Transmitted Drug Resistance in Antiretroviral Therapy-Naive Persons With Acute/Early/Primary HIV Infection: A Systematic Review and Meta-Analysis
- PMID: 34899288
- PMCID: PMC8652085
- DOI: 10.3389/fphar.2021.718763
Transmitted Drug Resistance in Antiretroviral Therapy-Naive Persons With Acute/Early/Primary HIV Infection: A Systematic Review and Meta-Analysis
Abstract
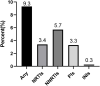
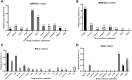
Background: The widespread use of antiretroviral therapy (ART) has raised concerns about the emergence of HIV transmitted drug resistance (TDR). Acute HIV infection (AHI) was the most appropriate time to detect the spread of TDR. In this meta-analysis, our purpose was to evaluate the level of TDR in ART-naive patients with primary HIV infection (PHI)/AHI/early HIV infection (EHI) and to describe the critical drug-resistant mutations. Methods: We systematically searched the literature between January 1, 2008, and April 30, 2021, in PubMed, Web of Science, Embase, and the Cochrane Library. To evaluate the overall prevalence of TDR, we extracted raw data and analyzed prevalence estimates using Stata SE. Results: The data of this meta-analysis come from 12 observational studies, covering 3,558 ART-naive individuals with PHI, AHI, or EHI. The overall prevalence of HIV-TDR is 9.3% (95% CI: 6.8%-11.8%, I2 = 81.1%, in 11 studies). The prevalence of resistance by drug class is the highest for the nonnucleoside reverse transcriptase inhibitors (NNRTIs) at 5.7% (95% CI: 2.9%-8.5%, I2 = 96.6%, in 11 studies), followed by nucleoside reverse transcriptase inhibitors (NRTIs) at 3.4% (95% CI: 1.8%-5.0%, I2 = 86.3%, in 10 studies) and protease inhibitors (PIs) at 3.3% (95% CI: 2.7%-3.9%, I2 = 15.6%, in 10 studies). The prevalence of TDR to integrase inhibitors (INIs) is 0.3% (95% CI: 0.1%-0.7%, I2 = 95.9%, in three studies), which is the lowest among all antiretroviral drugs. Conclusion: The overall prevalence of TDR is at a moderate level among AHI patients who have never received ART. This emphasizes the importance of baseline drug resistance testing for public health surveillance and guiding the choice of ART. In addition, the prevalence of TDR to NNRTIs is the highest, while the TDR to INIs is the lowest. This may guide the selection of clinical antiretroviral drugs.
Keywords: AIDS; HIV; acute infection; early infection; primary infection; transmitted drug resistance.
Copyright © 2021 Guo, Wu, Zhang, Liu, Li, Gao, Zhang, Wu, Chen and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- AIDS and Hepatitis C Professional Group, S. o. I. D. (2018). Chinese Medical Association;Chinese Center for Disease Control and Prevention: [Chinese Guidelines for Diagnosis and Treatment of HIV/AIDS (2018)]. Zhonghua Nei Ke Za Zhi 57 (12), 867–884. 10.3760/cma.j.issn.0578-1426.2018.12.002 - DOI - PubMed
-
- Ambrosioni J., Sued O., Nicolas D., Parera M., López-Diéguez M., Romero A., et al. (2015). Trends in Transmission of Drug Resistance and Prevalence of Non-B Subtypes in Patients with Acute or Recent HIV-1 Infection in Barcelona in the Last 16 Years (1997-2012). PLoS One 10 (6), e0125837. 10.1371/journal.pone.0125837 - DOI - PMC - PubMed
-
- Ananworanich J., Sirivichayakul S., Pinyakorn S., Crowell T. A., Trichavaroj R., Weerayingyong J., et al. (2015). High Prevalence of Transmitted Drug Resistance in Acute HIV-Infected Thai Men Who Have Sex with Men. J. Acquir Immune Defic Syndr. 68 (4), 481–485. 10.1097/QAI.0000000000000502 - DOI - PubMed
-
- Ávila-Ríos S., García-Morales C., Matías-Florentino M., Romero-Mora K. A., Tapia-Trejo D., Quiroz-Morales V. S., et al. (2016). Pretreatment HIV-Drug Resistance in Mexico and its Impact on the Effectiveness of First-Line Antiretroviral Therapy: a Nationally Representative 2015 WHO Survey. Lancet HIV 3 (12), e579–e591. 10.1016/s2352-3018(16)30119-9 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

