Roles of Macrophages in Atherogenesis
- PMID: 34899348
- PMCID: PMC8660976
- DOI: 10.3389/fphar.2021.785220
Roles of Macrophages in Atherogenesis
Abstract
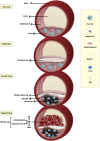
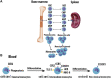
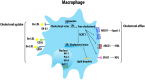
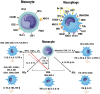
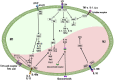
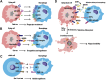
Atherosclerosis is a chronic inflammatory disease that may ultimately lead to local proteolysis, plaque rupture, and thrombotic vascular disease, resulting in myocardial infarction, stroke, and sudden cardiac death. Circulating monocytes are recruited to the arterial wall in response to inflammatory insults and differentiate into macrophages which make a critical contribution to tissue damage, wound healing, and also regression of atherosclerotic lesions. Within plaques, macrophages take up aggregated lipoproteins which have entered the vessel wall to give rise to cholesterol-engorged foam cells. Also, the macrophage phenotype is influenced by various stimuli which affect their polarization, efferocytosis, proliferation, and apoptosis. The heterogeneity of macrophages in lesions has recently been addressed by single-cell sequencing techniques. This article reviews recent advances regarding the roles of macrophages in different stages of disease pathogenesis from initiation to advanced atherosclerosis. Macrophage-based therapies for atherosclerosis management are also described.
Keywords: atherosclerosis; biomarker; inflammation; lipid; macrophages; plaque.
Copyright © 2021 Farahi, Sinha and Lusis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures