Differential Autophagy Response in Men and Women After Muscle Damage
- PMID: 34899384
- PMCID: PMC8652069
- DOI: 10.3389/fphys.2021.752347
Differential Autophagy Response in Men and Women After Muscle Damage
Abstract
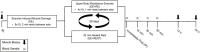
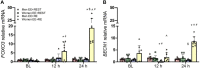
Following muscle damage, autophagy is crucial for muscle regeneration. Hormones (e.g., testosterone, cortisol) regulate this process and sex differences in autophagic flux exist in the basal state. However, to date, no study has examined the effect of a transient hormonal response following eccentric exercise-induced muscle damage (EE) between untrained young men and women. Untrained men (n = 8, 22 ± 3 years) and women (n = 8, 19 ± 1 year) completed two sessions of 80 unilateral maximal eccentric knee extensions followed by either upper body resistance exercise (RE; designed to induce a hormonal response; EE + RE) or a time-matched rest period (20 min; EE + REST). Vastus lateralis biopsy samples were collected before (BL), and 12 h, and 24 h after RE/REST. Gene and protein expression levels of selective markers for autophagic initiation signaling, phagophore initiation, and elongation/sequestration were determined. Basal markers of autophagy were not different between sexes. For EE + RE, although initiation signaling (FOXO3) and autophagy-promoting (BECN1) genes were greater (p < 0.0001; 12.4-fold, p = 0.0010; 10.5-fold, respectively) for women than men, autophagic flux (LC3-II/LC3-I protein ratio) did not change for women and was lower (p < 0.0001 3.0-fold) than men. Furthermore, regardless of hormonal changes, LC3-I and LC3-II protein content decreased (p = 0.0090; 0.547-fold, p = 0.0410; 0.307-fold, respectively) for men suggesting increased LC3-I lipidation and autophagosome degradation whereas LC3-I protein content increased (p = 0.0360; 1.485-fold) for women suggesting decreased LC3-I lipidation. Collectively, our findings demonstrated basal autophagy was not different between men and women, did not change after EE alone, and was promoted with the acute hormonal increase after RE only in men but not in women. Thus, the autophagy response to moderate muscle damage is promoted by RE-induced hormonal changes in men only.
Keywords: LC3-II/LC3-I ratio; cortisol; growth hormone; macroautophagy; sex dimorphism.
Copyright © 2021 Luk, Appell, Levitt, Jiwan and Vingren.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Sex-specific mitochondrial dynamics and mitophagy response to muscle damage.Physiol Rep. 2022 May;10(10):e15230. doi: 10.14814/phy2.15230. Physiol Rep. 2022. PMID: 35611770 Free PMC article.
-
Resistance exercise-induced hormonal response promotes satellite cell proliferation in untrained men but not in women.Am J Physiol Endocrinol Metab. 2019 Aug 1;317(2):E421-E432. doi: 10.1152/ajpendo.00473.2018. Epub 2019 Jun 25. Am J Physiol Endocrinol Metab. 2019. PMID: 31237450 Clinical Trial.
-
Role of AMPK in regulation of LC3 lipidation as a marker of autophagy in skeletal muscle.Cell Signal. 2016 Jun;28(6):663-74. doi: 10.1016/j.cellsig.2016.03.005. Epub 2016 Mar 12. Cell Signal. 2016. PMID: 26976209
-
Exercise-mediated modulation of autophagy in skeletal muscle.Scand J Med Sci Sports. 2018 Mar;28(3):772-781. doi: 10.1111/sms.12945. Epub 2017 Aug 4. Scand J Med Sci Sports. 2018. PMID: 28685860 Review.
-
Influence of Normal Aging on Brain Autophagy: A Complex Scenario.Front Aging Neurosci. 2019 Mar 11;11:49. doi: 10.3389/fnagi.2019.00049. eCollection 2019. Front Aging Neurosci. 2019. PMID: 30914945 Free PMC article. Review.
Cited by
-
Sex-specific mechanisms in vascular aging: exploring cellular and molecular pathways in the pathogenesis of age-related cardiovascular and cerebrovascular diseases.Geroscience. 2025 Feb;47(1):301-337. doi: 10.1007/s11357-024-01489-2. Epub 2025 Jan 3. Geroscience. 2025. PMID: 39754010 Free PMC article. Review.
-
Resistance exercise-induced circulating factors influence the damaged skeletal muscle proteome in a sex-dependent manner.Physiol Rep. 2025 Apr;13(7):e70291. doi: 10.14814/phy2.70291. Physiol Rep. 2025. PMID: 40223391 Free PMC article.
-
Characterizing 24-Hour Skeletal Muscle Gene Expression Alongside Metabolic and Endocrine Responses Under Diurnal Conditions.J Clin Endocrinol Metab. 2025 Mar 17;110(4):e1017-e1030. doi: 10.1210/clinem/dgae350. J Clin Endocrinol Metab. 2025. PMID: 38779872 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

