Eph-ephrin Signaling Affects Eye Lens Fiber Cell Intracellular Voltage and Membrane Conductance
- PMID: 34899394
- PMCID: PMC8656704
- DOI: 10.3389/fphys.2021.772276
Eph-ephrin Signaling Affects Eye Lens Fiber Cell Intracellular Voltage and Membrane Conductance
Abstract
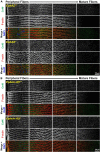
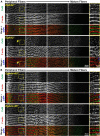
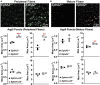
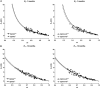
The avascular eye lens generates its own microcirculation that is required for maintaining lifelong lens transparency. The microcirculation relies on sodium ion flux, an extensive network of gap junction (GJ) plaques between lens fiber cells and transmembrane water channels. Disruption of connexin proteins, the building blocks of GJs, or aquaporins, which make up water and adhesion channels, lead to lens opacification or cataracts. Recent studies have revealed that disruption of Eph-ephrin signaling, in particular the receptor EphA2 and the ligand ephrin-A5, in humans and mice lead to congenital and age-related cataracts. We investigated whether changes in lens transparency in EphA2 or ephrin-A5 knockout (-/-) mice is related to changes in GJ coupling and lens fluid and ion homeostasis. Immunostaining revealed changes in connexin 50 (Cx50) subcellular localization in EphA2 -/- peripheral lens fibers and alteration in aquaporin 0 (Aqp0) staining patterns in ephrin-A5 -/- and EphA2 -/- inner mature fiber cells. Surprisingly, there was no obvious change in GJ coupling in knockout lenses. However, there were changes in fiber cell membrane conductance and intracellular voltage in knockout lenses from 3-month-old mice. These knockout lenses displayed decreased conductance of mature fiber membranes and were hyperpolarized compared to control lenses. This is the first demonstration that the membrane conductance of lens fibers can be regulated. Together these data suggest that EphA2 may be needed for normal Cx50 localization to the cell membrane and that conductance of lens fiber cells requires normal Eph-ephrin signaling and water channel localization.
Keywords: EphA2; aquaporin; connexin; ephrin-A5; gap junction coupling; resistivity.
Copyright © 2021 Cheng, Gao, Sun and Mathias.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

