α-1 Adrenoceptor Activation in the Dorsal Raphe Nucleus Decreases Food Intake in Fasted Rats
- PMID: 34899395
- PMCID: PMC8656260
- DOI: 10.3389/fphys.2021.775070
α-1 Adrenoceptor Activation in the Dorsal Raphe Nucleus Decreases Food Intake in Fasted Rats
Abstract
The dorsal raphe (DR) nucleus is involved in a myriad of physiological functions, such as the control of sleep-wake cycle, motivation, pain, energy balance, and food intake. We have previously demonstrated that in ad libitum fed rats the intra-DR administration of phenylephrine, an α-1 receptor agonist, does not affect food intake, whereas clonidine, an α-2 receptor agonist, potently stimulates food intake. These results indicated that in fed rats an increased adrenergic tonus blocked food intake, since the activation of α-2 auto-receptors, which decreases pre-synaptic release of adrenaline/noradrenaline, affected food intake. Thus, in this study we assessed whether the response to adrenergic stimuli would differ after overnight fasting, a situation of low adrenergic activity in the DR. Intra-DR administration of adrenaline and noradrenaline blocked food intake evoked by overnight fasting. Similarly, phenylephrine administration decreased hunger-induced food intake. These changes in food intake were accompanied by changes in other behaviors, such as increased immobility time and feeding duration. On the other hand, intra-DR administration of clonidine did not affect food-intake or associated behaviors. These results further support the hypothesis that in fed animals, increased adrenergic tonus in DR neurons inhibiting feeding, while in fasted rats the adrenergic tonus decreases and favors food intake. These data indicate a possible mechanism through which adrenergic input to the DRN contributes to neurobiology of feeding.
Keywords: adrenergic receptor; dorsal raphe (DR); food intake; hunger; phenylephrine.
Copyright © 2021 Flores, Dos-Santos, Steinbach, Rodrigues-Santos, de Jesus, Antunes-Rodrigues and Paschoalini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

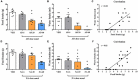
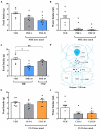

Similar articles
-
Injections of the α-2 adrenoceptor agonist clonidine into the dorsal raphe nucleus increases food intake in satiated rats.Neuropharmacology. 2021 Jan;182:108397. doi: 10.1016/j.neuropharm.2020.108397. Epub 2020 Nov 11. Neuropharmacology. 2021. PMID: 33188843
-
Tonic noradrenergic input to neurons in the dorsal raphe nucleus mediates food intake in male mice.Behav Brain Res. 2024 Mar 28;462:114872. doi: 10.1016/j.bbr.2024.114872. Epub 2024 Jan 23. Behav Brain Res. 2024. PMID: 38266779
-
Feeding behaviour after injection of α-adrenergic receptor agonists into the median raphe nucleus of food-deprived rats.Physiol Behav. 2012 Jan 18;105(2):220-9. doi: 10.1016/j.physbeh.2011.08.028. Epub 2011 Aug 31. Physiol Behav. 2012. PMID: 21903123
-
Evaluation of food intake and Fos expression in serotonergic neurons of raphe nuclei after intracerebroventricular injection of adrenaline in free-feeding rats.Brain Res. 2018 Jan 1;1678:153-163. doi: 10.1016/j.brainres.2017.10.021. Epub 2017 Oct 24. Brain Res. 2018. PMID: 29079504
-
Noradrenergic modulation of serotonin release in rat dorsal and median raphé nuclei via alpha(1) and alpha(2A) adrenoceptors.Neuropharmacology. 2001 Sep;41(4):433-42. doi: 10.1016/s0028-3908(01)00087-9. Neuropharmacology. 2001. PMID: 11543763
Cited by
-
Frontiers in metabolic physiology grand challenges.Front Physiol. 2022 Aug 10;13:879617. doi: 10.3389/fphys.2022.879617. eCollection 2022. Front Physiol. 2022. PMID: 36035475 Free PMC article. No abstract available.
-
α2-Adrenergic Receptors in Hypothalamic Dopaminergic Neurons: Impact on Food Intake and Energy Expenditure.Int J Mol Sci. 2025 Apr 10;26(8):3590. doi: 10.3390/ijms26083590. Int J Mol Sci. 2025. PMID: 40332078 Free PMC article.
References
LinkOut - more resources
Full Text Sources

