Appetite Regulation of TLR4-Induced Inflammatory Signaling
- PMID: 34899611
- PMCID: PMC8664591
- DOI: 10.3389/fendo.2021.777997
Appetite Regulation of TLR4-Induced Inflammatory Signaling
Abstract
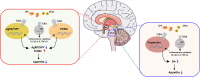
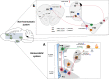
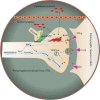
Appetite is the basis for obtaining food and maintaining normal metabolism. Toll-like receptor 4 (TLR4) is an important receptor expressed in the brain that induces inflammatory signaling after activation. Inflammation is considered to affect the homeostatic and non-homeostatic systems of appetite, which are dominated by hypothalamic and mesolimbic dopamine signaling. Although the pathological features of many types of inflammation are known, their physiological functions in appetite are largely unknown. This review mainly addresses several key issues, including the structures of the homeostatic and non-homeostatic systems. In addition, the mechanism by which TLR4-induced inflammatory signaling contributes to these two systems to regulate appetite is also discussed. This review will provide potential opportunities to develop new therapeutic interventions that control appetite under inflammatory conditions.
Keywords: SFAs; TLR4; dopamine system; hypothalamus; inflammation.
Copyright © 2021 Li, Jiang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The Role of Brain in Energy Balance.Adv Neurobiol. 2017;19:33-48. doi: 10.1007/978-3-319-63260-5_2. Adv Neurobiol. 2017. PMID: 28933060 Review.
-
The cannabinoid system: a role in both the homeostatic and hedonic control of eating?Br J Nutr. 2003 Oct;90(4):729-34. doi: 10.1079/bjn2003942. Br J Nutr. 2003. PMID: 13129440 Review.
-
[Appetite regulation: neuroendocrine basis and clinical approaches].Med Clin (Barc). 2012 Jun 16;139(2):70-5. doi: 10.1016/j.medcli.2011.11.024. Epub 2012 Jan 16. Med Clin (Barc). 2012. PMID: 22257602 Review. Spanish.
-
Oxytocin in the neural control of eating: At the crossroad between homeostatic and non-homeostatic signals.Neuropharmacology. 2020 Jul;171:108082. doi: 10.1016/j.neuropharm.2020.108082. Epub 2020 Apr 4. Neuropharmacology. 2020. PMID: 32259527 Review.
-
[Food intake regulation - 1st part].Vnitr Lek. 2013 Sep;59(9):808-17. Vnitr Lek. 2013. PMID: 24073953 Review. Czech.
Cited by
-
Quercetin Protects Against Global Cerebral ischemia‒reperfusion Injury by Inhibiting Microglial Activation and Polarization.J Inflamm Res. 2024 Feb 26;17:1281-1293. doi: 10.2147/JIR.S448620. eCollection 2024. J Inflamm Res. 2024. PMID: 38434580 Free PMC article.
-
Gut microbiota-derived gamma-aminobutyric acid improves host appetite by inhibiting satiety hormone secretion.mSystems. 2024 Oct 22;9(10):e0101524. doi: 10.1128/msystems.01015-24. Epub 2024 Sep 24. mSystems. 2024. PMID: 39315776 Free PMC article.
-
Plant-based dietary indices association with appetite, appetite regulating peptides and gut microbiota in healthy women: a cross-sectional study.Eur J Nutr. 2025 Apr 28;64(4):166. doi: 10.1007/s00394-025-03671-4. Eur J Nutr. 2025. PMID: 40293575
-
Proinflammatory cytokines interleukin-18 and interleukin-6 mediate anorexia induction by trichothecene deoxynivalenol and its congeners.Front Vet Sci. 2024 Dec 3;11:1521424. doi: 10.3389/fvets.2024.1521424. eCollection 2024. Front Vet Sci. 2024. PMID: 39691381 Free PMC article.
-
Subclinical Reactive Hypoglycemia with Low Glucose Effectiveness-Why We Cannot Stop Snacking despite Gaining Weight.Metabolites. 2023 Jun 15;13(6):754. doi: 10.3390/metabo13060754. Metabolites. 2023. PMID: 37367911 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

