Exploring the Prognostic Value, Immune Implication and Biological Function of H2AFY Gene in Hepatocellular Carcinoma
- PMID: 34899687
- PMCID: PMC8651705
- DOI: 10.3389/fimmu.2021.723293
Exploring the Prognostic Value, Immune Implication and Biological Function of H2AFY Gene in Hepatocellular Carcinoma
Abstract
Background: Hepatocellular carcinoma (HCC) is an extremely malignant cancer with poor survival. H2AFY gene encodes for a variant of H2A histone, and it has been found to be dysregulated in various tumors. However, the clinical value, biological functions and correlations with immune infiltration of H2AFY in HCC remain unclear.
Methods: We analyzed the expression and clinical significance of H2AFY in HCC using multiple databases, including Oncomine, HCCDB, TCGA, ICGC, and so on. The genetic alterations of H2AFY were analyzed by cBioPortal and COSMIC databases. Co-expression networks of H2AFY and its regulators were investigated by LinkedOmics. The correlations between H2AFY and tumor immune infiltration were explored using TIMER, TISIDB databases, and CIBERSORT method. Finally, H2AFY was knocked down with shRNA lentiviruses in HCC cell lines for functional assays in vitro.
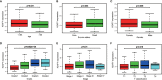
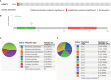
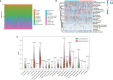
Results: H2AFY expression was upregulated in the HCC tissues and cells. Kaplan-Meier and Cox regression analyses revealed that high H2AFY expression was an independent prognostic factor for poor survival in HCC patients. Functional network analysis indicated that H2AFY and its co-expressed genes regulates cell cycle, mitosis, spliceosome and chromatin assembly through pathways involving many cancer-related kinases and E2F family. Furthermore, we observed significant correlations between H2AFY expression and immune infiltration in HCC. H2AFY knockdown suppressed the cell proliferation and migration, promoted cycle arrest, and apoptosis of HCC cells in vitro.
Conclusion: Our study revealed that H2AFY is a potential biomarker for unfavorable prognosis and correlates with immune infiltration in HCC.
Keywords: H2AFY; biomarker; hepatocellular carcinoma; immune infiltration; prognosis.
Copyright © 2021 Huang, Huang, Ma, Wang, Wang, Xiao, Qin, Li and Yuan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

