Monomeric IgA Antagonizes IgG-Mediated Enhancement of DENV Infection
- PMID: 34899736
- PMCID: PMC8654368
- DOI: 10.3389/fimmu.2021.777672
Monomeric IgA Antagonizes IgG-Mediated Enhancement of DENV Infection
Abstract
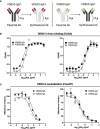
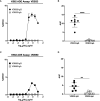
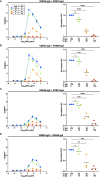
Dengue virus (DENV) is a prevalent human pathogen, infecting approximately 400 million individuals per year and causing symptomatic disease in approximately 100 million. A distinct feature of dengue is the increased risk for severe disease in some individuals with preexisting DENV-specific immunity. One proposed mechanism for this phenomenon is antibody-dependent enhancement (ADE), in which poorly-neutralizing IgG antibodies from a prior infection opsonize DENV to increase infection of Fc gamma receptor-bearing cells. While IgM and IgG are the most commonly studied DENV-reactive antibody isotypes, our group and others have described the induction of DENV-specific serum IgA responses during dengue. We hypothesized that monomeric IgA would be able to neutralize DENV without the possibility of ADE. To test this, we synthesized IgG and IgA versions of two different DENV-reactive monoclonal antibodies. We demonstrate that isotype-switching does not affect the antigen binding and neutralization properties of the two mAbs. We show that DENV-reactive IgG, but not IgA, mediates ADE in Fc gamma receptor-positive K562 cells. Furthermore, we show that IgA potently antagonizes the ADE activity of IgG. These results suggest that levels of DENV-reactive IgA induced by DENV infection might regulate the overall IgG mediated ADE activity of DENV-immune plasma in vivo, and may serve as a predictor of disease risk.
Keywords: ADE; DENV; Dengue; IgA; antibody dependent enhancement.
Copyright © 2021 Wegman, Fang, Rothman, Thomas, Endy, McCracken, Currier, Friberg, Gromowski and Waickman.
Conflict of interest statement
Authors ADW, MM, JC, HF, GG, and ATW are co-inventors on the provisional patent “IgA monoclonal antibodies as a prophylactic and therapeutic treatment for acute flavivirus infection”. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

