Neutrophil Extracellular Traps Promote T Cell Exhaustion in the Tumor Microenvironment
- PMID: 34899751
- PMCID: PMC8652262
- DOI: 10.3389/fimmu.2021.785222
Neutrophil Extracellular Traps Promote T Cell Exhaustion in the Tumor Microenvironment
Abstract
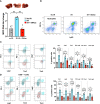
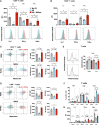
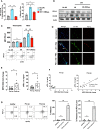
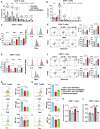
While neutrophil extracellular traps (NETs) are important for directly promoting cancer growth, little is known about their impact on immune cells within the tumor microenvironment (TME). We hypothesize that NETs can directly interact with infiltrating T cells to promote an immunosuppressive TME. Herein, to induce a NET-rich TME, we performed liver Ischemia/Reperfusion (I/R) in an established cancer metastasis model or directly injected NETs in subcutaneous tumors. In this NET-rich TME, the majority of CD4+ and CD8+ tumor infiltrating lymphocytes expressed multiple inhibitory receptors, in addition these cells showed a functional and metabolic exhausted phenotype. Targeting of NETs in vivo by treating mice with DNAse lead to decreased tumor growth, decreased NET formation and higher levels of functioning T cells. In vitro, NETs contained the immunosuppressive ligand PD-L1 responsible for T cell exhaustion and dysfunction; an effect abrogated by using PD-L1 KO NETs or culturing NETs with PD-1 KO T cells. Furthermore, we found elevated levels of sPDL-1 and MPO-DNA, a NET marker, in the serum of patients undergoing surgery for colorectal liver metastases resection. Neutrophils isolated from patients after surgery were primed to form NETs and induced exhaustion and dysfunction of human CD4+ and CD8+ T cells. We next targeted PD-L1 in vivo by injecting a blocking antibody during liver I/R. A single dose of anti-PD-L1 during surgery lead to diminished tumors at 3 weeks and functional T cells in the TME. Our data thus reveal that NETs have the capability of suppressing T cell responses through metabolic and functional exhaustion and thereby promote tumor growth. Furthermore, targeting of PD-L1 containing NETs at time of surgery with DNAse or anti-PD-L1 lead to diminished tumor growth, which represents a novel and viable strategy for sustaining immune competence within the TME.
Keywords: PD-L1; T cell dysfunction; T cell exhaustion; neutrophil extracellular traps; program death-ligand 1; tumor microenvironment.
Copyright © 2021 Kaltenmeier, Yazdani, Morder, Geller, Simmons and Tohme.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

