Recent Advances in Developmental Hematopoiesis: Diving Deeper With New Technologies
- PMID: 34899758
- PMCID: PMC8652083
- DOI: 10.3389/fimmu.2021.790379
Recent Advances in Developmental Hematopoiesis: Diving Deeper With New Technologies
Abstract
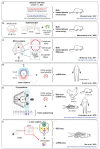
The journey of a hematopoietic stem cell (HSC) involves the passage through successive anatomical sites where HSCs are in direct contact with their surrounding microenvironment, also known as niche. These spatial and temporal cellular interactions throughout development are required for the acquisition of stem cell properties, and for maintaining the HSC pool through balancing self-renewal, quiescence and lineage commitment. Understanding the context and consequences of these interactions will be imperative for our understanding of HSC biology and will lead to the improvement of in vitro production of HSCs for clinical purposes. The aorta-gonad-mesonephros (AGM) region is in this light of particular interest since this is the cradle of HSC emergence during the embryonic development of all vertebrate species. In this review, we will focus on the developmental origin of HSCs and will discuss the novel technological approaches and recent progress made to identify the cellular composition of the HSC supportive niche and the underlying molecular events occurring in the AGM region.
Keywords: aorta-gonad-mesonephros; embryo; hematopoietic stem cells; hemogenic endothelium; microenvironment; niche; single cell RNA sequencing; tomography sequencing.
Copyright © 2021 Weijts, Yvernogeau and Robin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

