How Times Have Changed! A Cornucopia of Antigens for Membranous Nephropathy
- PMID: 34899763
- PMCID: PMC8662735
- DOI: 10.3389/fimmu.2021.800242
How Times Have Changed! A Cornucopia of Antigens for Membranous Nephropathy
Abstract
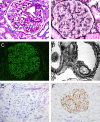
The identification of the major target antigen phospholipase A2 receptor (PLA2R) in the majority of primary (idiopathic) cases of membranous nephropathy (MN) has been followed by the rapid identification of numerous minor antigens that appear to define phenotypically distinct forms of disease. This article serves to review all the known antigens that have been shown to localize to subepithelial deposits in MN, as well as the distinctive characteristics associated with each subtype of MN. We will also shed light on the novel proteomic approaches that have allowed identification of the most recent antigens. The paradigm of an antigen normally expressed on the podocyte cell surface leading to in-situ immune complex formation, complement activation, and subsequent podocyte injury will be discussed and challenged in light of the current repertoire of multiple MN antigens. Since disease phenotypes associated with each individual target antigens can often blur the distinction between primary and secondary disease, we encourage the use of antigen-based classification of membranous nephropathy.
Keywords: antigen; autoimmune profiling; epitope spreading; mass spectrometry; membranous lupus nephritis; membranous nephropathy; serologic testing.
Copyright © 2021 Caza, Al-Rabadi and Beck.
Conflict of interest statement
LB reports being a co-inventor on the patent “Diagnostics for Membranous Nephropathy” and receives royalty income through his employer Boston University. LB has served on advisory boards on the topic of MN and other glomerular diseases for Visterra, Ionis, Alexion and Novartis, and receives royalties from UpToDate for topics related to MN. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

