Sinomenine ester derivative inhibits glioblastoma by inducing mitochondria-dependent apoptosis and autophagy by PI3K/AKT/mTOR and AMPK/mTOR pathway
- PMID: 34900530
- PMCID: PMC8642618
- DOI: 10.1016/j.apsb.2021.05.027
Sinomenine ester derivative inhibits glioblastoma by inducing mitochondria-dependent apoptosis and autophagy by PI3K/AKT/mTOR and AMPK/mTOR pathway
Abstract
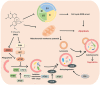
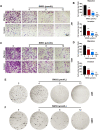
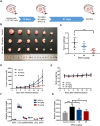
Glioblastoma multiforme (GBM) in the central nervous system is the most lethal advanced glioma and currently there is no effective treatment for it. Studies of sinomenine, an alkaloid from the Chinese medicinal plant, Sinomenium acutum, showed that it had inhibitory effects on several kinds of cancer. Here, we synthesized a sinomenine derivative, sino-wcj-33 (SW33), tested it for antitumor activity on GBM and explored the underlying mechanism. SW33 significantly inhibited proliferation and colony formation of GBM and reduced migration and invasion of U87 and U251 cells. It also arrested the cell cycle at G2/M phase and induced mitochondria-dependent apoptosis. Differential gene enrichment analysis and pathway validation showed that SW33 exerted anti-GBM effects by regulating PI3K/AKT and AMPK signaling pathways and significantly suppressed tumorigenicity with no obvious adverse effects on the body. SW33 also induced autophagy through the PI3K/AKT/mTOR and AMPK/mTOR pathways. Thus, SW33 appears to be a promising drug for treating GBM effectively and safely.
Keywords: Anti-inflammation; Apoptosis; Autophagy; G2/M phase; GBM; SW33; Safety; mTOR.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures





References
-
- Ostrom Q.T., Gittleman H., Stetson L., Virk S.M., Barnholtz-Sloan J.S. Epidemiology of gliomas. Cancer Treat Res. 2015;163:1–14. - PubMed
-
- Bush N.A., Chang S.M., Berger M.S. Current and future strategies for treatment of glioma. Neurosurg Rev. 2017;40:1–14. - PubMed
-
- Alexander B.M., Cloughesy T.F. Adult glioblastoma. J Clin Oncol. 2017;35:2402–2409. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

