Protocatechuic aldehyde protects cardiomycoytes against ischemic injury via regulation of nuclear pyruvate kinase M2
- PMID: 34900536
- PMCID: PMC8642444
- DOI: 10.1016/j.apsb.2021.03.021
Protocatechuic aldehyde protects cardiomycoytes against ischemic injury via regulation of nuclear pyruvate kinase M2
Abstract
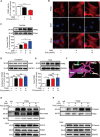
Rescuing cells from stress damage emerges a potential therapeutic strategy to combat myocardial infarction. Protocatechuic aldehyde (PCA) is a major phenolic acid in Chinese herb Danshen (Salvia miltiorrhiza root). This study investigated whether PCA regulated nuclear pyruvate kinase isoform M2 (PKM2) function to protect cardiomyocytes. In rats subjected to isoprenaline, PCA attenuated heart injury and protected cardiomyocytes from apoptosis. Through DARTS and CETSA assays, we identified that PCA bound and promoted PKM2 nuclear translocation in cardiomyocytes exposed to oxygen/glucose deprivation (OGD). In the nucleus, PCA increased the binding of PKM2 to β-catenin via preserving PKM2 acetylation, and the complex, in cooperation with T-cell factor 4 (TCF4), was required for transcriptional induction of genes encoding anti-apoptotic proteins, contributing to rescuing cardiomyocyte survival. In addition, PCA ameliorated mitochondrial dysfunction and prevented mitochondrial apoptosis dependent on PKM2. Consistently, PCA increased the binding of PKM2 to β-catenin, improved heart contractive function, normalized heart structure and attenuated oxidative damage in mice subjected to artery ligation, but the protective effects were lost in Pkm2-deficient heart. Together, we showed that PCA regulated nuclear PKM2 function to rescue cardiomyocyte survival via β-catenin/TCF4 signaling cascade, suggesting the potential of pharmacological intervention of PKM2 shuttle to protect the heart.
Keywords: Apoptosis; CETSA, cellular thermal shift assay; CK-MB, creatine kinase isoenzyme-MB; DARTS, drug affinity responsive target stability; Heart ischemia; ISO, isoprenaline; LDH, lactate dehydrogenase; Mitochondrial damage; Myocardial infarction; NRVMs, neonatal rat ventricular myocytes; Nuclear translocation; OGD, oxygen and glucose deprivation; PCA, protocatechuic aldehyde; PKM2; PKM2, pyruvate kinase isoform M2; Protocatechuic aldehyde; ROS, reactive oxygen species; TCF4; TCF4, T-cell factor 4; TUNEL, deoxynucleotidyl transferase-mediated dUTP nick end-labeling; shRNA, short hairpin RNA; β-Catenin.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures





Similar articles
-
Protocatechuic aldehyde protects against isoproterenol-induced cardiac hypertrophy via inhibition of the JAK2/STAT3 signaling pathway.Naunyn Schmiedebergs Arch Pharmacol. 2018 Dec;391(12):1373-1385. doi: 10.1007/s00210-018-1556-7. Epub 2018 Aug 21. Naunyn Schmiedebergs Arch Pharmacol. 2018. PMID: 30132020
-
Dihydrotanshinone I preconditions myocardium against ischemic injury via PKM2 glutathionylation sensitive to ROS.Acta Pharm Sin B. 2023 Jan;13(1):113-127. doi: 10.1016/j.apsb.2022.07.006. Epub 2022 Jul 16. Acta Pharm Sin B. 2023. PMID: 36815040 Free PMC article.
-
Protocatechuic aldehyde prevents ischemic injury by attenuating brain microvascular endothelial cell pyroptosis via lncRNA Xist.Phytomedicine. 2022 Jan;94:153849. doi: 10.1016/j.phymed.2021.153849. Epub 2021 Nov 2. Phytomedicine. 2022. PMID: 34775360
-
ML285 affects reactive oxygen species’ inhibition of pyruvate kinase M2.2012 Aug 3 [updated 2013 May 8]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2012 Aug 3 [updated 2013 May 8]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 23885364 Free Books & Documents. Review.
-
Antioxidant Effects of Protocatechuic Acid and Protocatechuic Aldehyde: Old Wine in a New Bottle.Evid Based Complement Alternat Med. 2021 Nov 8;2021:6139308. doi: 10.1155/2021/6139308. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34790246 Free PMC article. Review.
Cited by
-
The Role of PKM2 in Multiple Signaling Pathways Related to Neurological Diseases.Mol Neurobiol. 2024 Aug;61(8):5002-5026. doi: 10.1007/s12035-023-03901-y. Epub 2023 Dec 29. Mol Neurobiol. 2024. PMID: 38157121 Review.
-
Mechanism for effect of tanshinone IIA on alleviating cardiomyocyte injury induced by oxygen glucose deprivation.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023 Mar 28;48(3):369-375. doi: 10.11817/j.issn.1672-7347.2023.220173. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37164920 Free PMC article. Chinese, English.
-
Emodin mitigates rheumatoid arthritis through direct binding to TNF-α.Front Pharmacol. 2025 Feb 26;16:1520281. doi: 10.3389/fphar.2025.1520281. eCollection 2025. Front Pharmacol. 2025. PMID: 40078278 Free PMC article.
-
The role of glycolytic metabolic pathways in cardiovascular disease and potential therapeutic approaches.Basic Res Cardiol. 2023 Nov 8;118(1):48. doi: 10.1007/s00395-023-01018-w. Basic Res Cardiol. 2023. PMID: 37938421 Free PMC article. Review.
-
AARS2 ameliorates myocardial ischemia via fine-tuning PKM2-mediated metabolism.Elife. 2025 May 15;13:RP99670. doi: 10.7554/eLife.99670. Elife. 2025. PMID: 40371904 Free PMC article.
References
-
- Cheng Y., Feng Y., Xia Z., Li X., Rong J. ω-Alkynyl arachidonic acid promotes anti-inflammatory macrophage M2 polarization against acute myocardial infarction via regulating the cross-talk between PKM2, HIF-1α and iNOS. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:1595–1605. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

