Immune-Related Adverse Events in PD-1 Treated Melanoma and Impact Upon Anti-Tumor Efficacy: A Real World Analysis
- PMID: 34900695
- PMCID: PMC8662734
- DOI: 10.3389/fonc.2021.749064
Immune-Related Adverse Events in PD-1 Treated Melanoma and Impact Upon Anti-Tumor Efficacy: A Real World Analysis
Abstract
Background: Anti-PD-1 immune checkpoint inhibitor (ICI) therapy has revolutionized the treatment of melanoma by producing durable long-term responses in a subset of patients. ICI-treated patients develop unique toxicities - immune related adverse events (irAEs) - that arise from unrestrained immune activation. The link between irAE development and clinical outcome in melanoma and other cancers is inconsistent; and little data exists on the occurrence of multiple irAEs. We sought to characterize development of single and multiple irAEs, and association of irAE(s) development with clinical variables and impact upon outcomes in advanced melanoma patients treated with anti-PD-1 ICIs.
Methods: We conducted a retrospective study of 190 patients with metastatic melanoma treated with single-agent anti-PD-1 ICI therapy between June 2014 and August 2020 at a large integrated network cancer center identified through retrospective review of pharmacy records. irAEs were graded based on the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
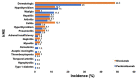
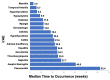
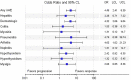
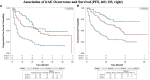
Results: 190 patients were evaluated of whom 114 patients (60.0%) experienced ≥1 irAE, including 30 (15.8%) with grade 3/4 irAEs. The occurrence of any irAE was strongly associated with the development of investigator-assessed response to anti-PD-1 therapy (p < 0.0001); whether evaluated by current (p=0.0082) or best (p=0.0001) response. In patients with ≥2 irAEs, distinct patterns were observed. Median progression-free survival (PFS) and overall survival (OS) were greater in those with any irAE compared to those without (PFS, 28 months vs. 5 months, p < 0.0001; OS, not reached vs. 9 months, p < 0.0001). Development of ≥2 irAEs had a trend towards improved PFS and OS compared to those who developed a single irAE, although this did not reach statistical significance (p=0.2555, PFS; p=0.0583, OS). Obesity but not age or gender was distinctly associated with irAE development.
Conclusions: In this study, we demonstrated that irAE occurrence was significantly associated with response to anti-PD-1 therapy and improved PFS/OS. Those who developed multiple irAEs had a trend towards improved PFS and OS compared to those who developed only a single irAE. Increased BMI but neither age nor gender were associated with irAE development. Distinct patterns of irAEs observed suggest shared etiopathogenetic mechanisms.
Keywords: CTLA-4; PD-1; autoimmune; immune related adverse events; immunotherapy; irAE; melanoma; metastatic.
Copyright © 2021 Bastacky, Wang, Fortman, Rahman, Mascara, Brenner, Najjar, Luke, Kirkwood, Zarour and Davar.
Conflict of interest statement
YN: Research Funding (Merck, Pfizer, and Bristol-Myers Squibb). JL: Stock and Other Ownership Interests (RefleXion); Consulting/Advisory Role (7 Hills, Abbvie, Alnylam, Actym, Alphamab Oncology, Arch Oncology, Array, Bayer, Bristol-Myers Squibb, Checkmate Pharmaceuticals, Cstone, Eisai, EMD Serono, Flame,Fstar, Gilead, Kadmon, KSQ, Knaph, Janssen, Immunocore, Inzen, Macrogenics,Mavu, Merck, Mersana, Nektar, Novartis, Onc.AI, Pfizer, Pyxis, Regeneron, Ribon, Rubius, Silicon, Synlogic, TRex, Tempest, Werewolf, Xilio, Xencor); Research Funding (AbbVie, Agios, Array, Astellas, Bristol-Myers Squibb, Corvus, EMD Serono, Genmab, Ikena, Immatics, Incyte, Kadmon, KAHR, MAcrogenics, Merck, Moderna, Nektar, Numab, Replimmune, Rubius, Spring Bank, Synlogic, Takeda, Trishula, Tizona, Xencor). JK: Consulting/Advisory Role (Amgen, Bristol-Myers Squibb, Checkmate Pharmaceuticals, and Novartis); Research Funding (Amgen, Bristol-Myers Squibb, Castle Biosciences, Checkmate Pharmaceuticals, Immunocore LLC, Iovance, and Novartis). HZ: Consulting/Advisory Role (Bristol Myers Squibb, Checkmate Pharmaceuticals, GlaxoSmithKline/Tesaro and Vedanta Biosciences); Research Funding (Bristol Myers Squibb, Checkmate Pharmaceuticals, and GlaxoSmithKline/Tesaro). DD: Consulting/Advisory Role (Ascendis Pharma, Bristol Myers Squibb, Checkmate Pharmaceuticals, Shionogi, Vedanta Biosciences); Research Funding (Arcus, Checkmate Pharmaceuticals, Cellsight Technologies, GlaxoSmithKline/Tesaro, Merck Inc.). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, et al. . Differential Expression of Immune-Regulatory Genes Associated With PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin Cancer Res (2015) 21(17):3969–76. doi: 10.1158/1078-0432.CCR-15-0244 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

