Endocrine Regulation of Extra-skeletal Organs by Bone-derived Secreted Protein and the effect of Mechanical Stimulation
- PMID: 34901023
- PMCID: PMC8652208
- DOI: 10.3389/fcell.2021.778015
Endocrine Regulation of Extra-skeletal Organs by Bone-derived Secreted Protein and the effect of Mechanical Stimulation
Abstract
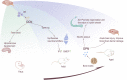
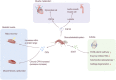
Bone serves as the support for body and provide attachment points for the muscles. The musculoskeletal system is the basis for the human body to complete exercise. Studies believe that bone is not only the basis for constructing structures, but also participates in the regulation of organs outside bone. The realization of this function is closely related to the protein secreted by bone. Whether bone can realize their positions in the human body is also related to their secretion. Bone-derived proteins provide a medium for the targeted regulation of bones on organs, making the role of bone in human body more profound and concrete. Mechanical stimulation effects the extra-skeletal organs by causing quantitative changes in bone-derived factors. When bone receives mechanical stimulation, the nichle of bone responds, and the secretion of various factors changes. However, whether the proteins secreted by bone can interfere with disease requires more research. In this review article, we will first introduce the important reasons and significance of the in-depth study on bone-derived secretory proteins, and summarize the locations, structures and functions of these proteins. These functions will not only focus on the bone metabolism process, but also be reflected in the cross-organ regulation. We specifically explain the role of typical bone-derived secretory factors such as osteocalcin (OCN), osteopontin (OPN), sclerostin (SOST) and fibroblast growth factor 23 (FGF23) in different organs and metabolic processes, then establishing the relationship between them and diseases. Finally, we will discuss whether exercise or mechanical stimulation can have a definite effect on bone-derived secretory factors. Understanding their important role in cross-organ regulation is of great significance for the treatment of diseases, especially for the elderly people with more than one basic disease.
Keywords: bone; cross-organ regulation; endocrine regulation; exercise prescription; mechanical stimulation; secreted protein.
Copyright © 2021 Du, Zhang, Wang, Zhao and Zou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Bone-derived factors mediate crosstalk between skeletal and extra-skeletal organs.Bone Res. 2025 Apr 30;13(1):49. doi: 10.1038/s41413-025-00424-1. Bone Res. 2025. PMID: 40307216 Free PMC article. Review.
-
Muscle, Bone, and Fat Crosstalk: the Biological Role of Myokines, Osteokines, and Adipokines.Curr Osteoporos Rep. 2020 Aug;18(4):388-400. doi: 10.1007/s11914-020-00599-y. Curr Osteoporos Rep. 2020. PMID: 32529456 Review.
-
Osteokines and the vasculature: a review of the in vitro effects of osteocalcin, fibroblast growth factor-23 and lipocalin-2.PeerJ. 2019 Jul 24;7:e7139. doi: 10.7717/peerj.7139. eCollection 2019. PeerJ. 2019. PMID: 31372314 Free PMC article.
-
[The regulation of various organs by osteoblasts].Clin Calcium. 2016 May;26(5):721-7. Clin Calcium. 2016. PMID: 27117618 Review. Japanese.
-
[Bone-derived hormones and their systemic regulation.].Clin Calcium. 2018;28(1):9-15. Clin Calcium. 2018. PMID: 29279421 Review. Japanese.
Cited by
-
The role of PIEZO ion channels in the musculoskeletal system.Am J Physiol Cell Physiol. 2023 Mar 1;324(3):C728-C740. doi: 10.1152/ajpcell.00544.2022. Epub 2023 Jan 30. Am J Physiol Cell Physiol. 2023. PMID: 36717101 Free PMC article. Review.
-
Sclerostin is a promising therapeutic target for oral inflammation and regenerative dentistry.J Transl Med. 2022 May 13;20(1):221. doi: 10.1186/s12967-022-03417-4. J Transl Med. 2022. PMID: 35562828 Free PMC article. Review.
-
Research advances in crosstalk between muscle and bone in osteosarcopenia (Review).Exp Ther Med. 2023 Mar 14;25(4):189. doi: 10.3892/etm.2023.11888. eCollection 2023 Apr. Exp Ther Med. 2023. PMID: 37021068 Free PMC article. Review.
-
Interrelationships among metabolic syndrome, bone-derived cytokines, and the most common metabolic syndrome-related diseases negatively affecting bone quality.Diabetol Metab Syndr. 2024 Sep 6;16(1):217. doi: 10.1186/s13098-024-01440-7. Diabetol Metab Syndr. 2024. PMID: 39238022 Free PMC article. Review.
-
Regulation of bone homeostasis: signaling pathways and therapeutic targets.MedComm (2020). 2024 Jul 24;5(8):e657. doi: 10.1002/mco2.657. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39049966 Free PMC article. Review.
References
-
- Asai S., Cao X., Yamauchi M., Funahashi K., Ishiguro N., Kambe F. (2009). Thyroid Hormone Non-genomically Suppresses Src Thereby Stimulating Osteocalcin Expression in Primary Mouse Calvarial Osteoblasts. Biochem. Biophysical Res. Commun. 387 (1), 92–96. Epub 2009/07/01. 10.1016/j.bbrc.2009.06.131 - DOI - PubMed
-
- Bai X., Miao D., Li J., Goltzman D., Karaplis A. C. (2004). Transgenic Mice Overexpressing Human Fibroblast Growth Factor 23 (R176Q) Delineate a Putative Role for Parathyroid Hormone in Renal Phosphate Wasting Disorders. Endocrinology 145 (11), 5269–5279. Epub 2004/07/31. 10.1210/en.2004-0233 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

