LigD: A Structural Guide to the Multi-Tool of Bacterial Non-Homologous End Joining
- PMID: 34901162
- PMCID: PMC8656161
- DOI: 10.3389/fmolb.2021.787709
LigD: A Structural Guide to the Multi-Tool of Bacterial Non-Homologous End Joining
Abstract
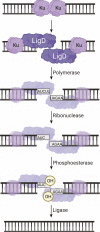
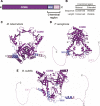
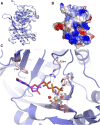
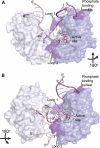
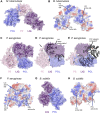
DNA double-strand breaks are the most lethal form of damage for living organisms. The non-homologous end joining (NHEJ) pathway can repair these breaks without the use of a DNA template, making it a critical repair mechanism when DNA is not replicating, but also a threat to genome integrity. NHEJ requires proteins to anchor the DNA double-strand break, recruit additional repair proteins, and then depending on the damage at the DNA ends, fill in nucleotide gaps or add or remove phosphate groups before final ligation. In eukaryotes, NHEJ uses a multitude of proteins to carry out processing and ligation of the DNA double-strand break. Bacterial NHEJ, though, accomplishes repair primarily with only two proteins-Ku and LigD. While Ku binds the initial break and recruits LigD, it is LigD that is the primary DNA end processing machinery. Up to three enzymatic domains reside within LigD, dependent on the bacterial species. These domains are a polymerase domain, to fill in nucleotide gaps with a preference for ribonucleotide addition; a phosphoesterase domain, to generate a 3'-hydroxyl DNA end; and the ligase domain, to seal the phosphodiester backbone. To date, there are no experimental structures of wild-type LigD, but there are x-ray and nuclear magnetic resonance structures of the individual enzymatic domains from different bacteria and archaea, along with structural predictions of wild-type LigD via AlphaFold. In this review, we will examine the structures of the independent domains of LigD from different bacterial species and the contributions these structures have made to understanding the NHEJ repair mechanism. We will then examine how the experimental structures of the individual LigD enzymatic domains combine with structural predictions of LigD from different bacterial species and postulate how LigD coordinates multiple enzymatic activities to carry out DNA double-strand break repair in bacteria.
Keywords: DNA double-strand break; LigD; ligase; non-homologous end joining; phosphoesterase; polymerase; protein structure and function.
Copyright © 2021 Amare, Mo, Khan, Sowa, Warner, Tetenych and Andres.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

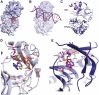

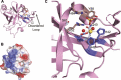
References
Publication types
LinkOut - more resources
Full Text Sources

