Developing Structure-Activity Relationships for N-Nitrosamine Activity
- PMID: 34901581
- PMCID: PMC8659209
- DOI: 10.1016/j.comtox.2021.100186
Developing Structure-Activity Relationships for N-Nitrosamine Activity
Abstract
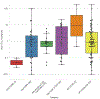
The detection of N-nitrosodimethylamine (NDMA) in several marketed drugs led regulatory agencies to require that N-nitrosamine risk assessments be performed on all marketed medical products [EMA/351053/2019 rev 1 (2019)]. Regulation of N-nitrosamine impurity levels in pharmaceutical drug substances and products is described in the ICH M7(R1) guideline where they are referred to as "cohort-of-concern" compounds as several are potent rodent carcinogens [Kroes et. al. 2004]. EMA, U.S. FDA and other regulatory agencies have set provisional acceptable daily intake limits for N-nitrosamines calculated from rodent carcinogenicity TD50 values for experimentally measured N-nitrosamines or the measured TD50 values of close analogs. The class-specific limit can be adjusted based upon a structure activity relationship analysis (SAR) and comparison with analogs having established carcinogenicity data [EMA/369136/2020, (2020)]. To investigate whether improvements in SARs can more accurately predict N-nitrosamine carcinogenic potency, an ad hoc workgroup of 23 companies and universities was established with the goals of addressing several scientific and regulatory issues including: reporting and review of N-nitrosamine mutagenicity and carcinogenicity reaction mechanisms, collection and review of available, public relevant experimental data, development of structure-activity relationships consistent with mechanisms for prediction of N-nitrosamine carcinogenic potency categories, and improved methods for calculating acceptable intake limits for N-nitrosamines based upon mechanistic analogs. Here we describe this collaboration and review our progress to date towards development of mechanistically based structure-activity relationships. We propose improving risk assessment of N-nitrosamines by first establishing the dominant reaction mechanism prior to retrieving an appropriate set of close analogs for use in read-across exercises.
Conflict of interest statement
Conflict of Interest The authors are employed full-time by their respective companies which develop software for the prediction of chemical toxicity as listed as their affiliations and have performed this work as part of their research tasks in collaboration with each other and the informal working group discussed; no product, commercial, market or strategic information has been shared in discussion, nor has it influenced this work. Declaration of interests The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Kevin P. Cross reports financial support was provided by National Institute of Environmental Health Sciences of the National Institutes of Health. Kevin P. Cross reports a relationship with Instem that includes: employment, equity or stocks, and travel reimbursement.
Figures
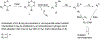


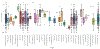


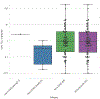

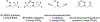


References
-
- European Medicines Agency (EMA), Temporary interim limits for NMBA, DIPNA, EIPNA, impurities in sartan blood pressure medicines. EMA/351053/2019 rev 1., 2019.
-
- Kroes R, Renwick AG, Cheeseman MA, Kleiner J, Mangelsdorf I, Piersma A, Schilter B, Schlatter J, Van Schothorst F, Vos JG, Würtzen G, Structure-based thresholds of toxicological concern (TTC): Guidance for application to substances present at low levels in the diet, Food Chem. Toxicol 42 (2004) 65–83. 10.1016/j.fct.2003.08.006. - DOI - PubMed
-
- SwissMedic, Potential nitrosamine contamination, (n.d.). https://www.swissmedic.ch/swissmedic/en/home/news/mitteilungen/aufforder... (accessed June 22, 2021).
-
- European Medicines Agency (EMA), Procedure under Article 5(3) of Regulation EC (No) 726/2004: Nitrosamine impurities in human medicinal products. Procedure number: EMEA/H/A-5(3)/1490. EMA/369136/2020, (2020).
-
- European Medicines Agency (EMA), Questions and answers for marketing authorisation holders/applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 referral on nitrosamine impurities in human medicinal products. EMA/409815/2020, 2020.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
