Exploring the Interactions of Ionic Liquids with Bio-Organic Amphiphiles Using Computational Approaches
- PMID: 34901596
- PMCID: PMC8655765
- DOI: 10.1021/acsomega.1c03864
Exploring the Interactions of Ionic Liquids with Bio-Organic Amphiphiles Using Computational Approaches
Abstract
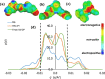
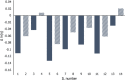
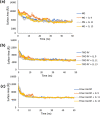
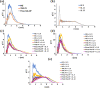
Bio-organic amphiphiles have been shown to effectively impart unique physicochemical properties to ionic liquids resulting in the formation of versatile hybrid composites. In this work, we utilized computational methods to probe the formation and properties of hybrids prepared by mixing three newly designed bio-organic amphiphiles with 14 ionic liquids containing cholinium or glycine betaine cations and a variety of anions. The three amphiphiles were designed such that they contain unique biological moieties found in nature by conjugating (a) malic acid with the amino acid glutamine, (b) thiomalic acid with the antiviral, antibacterial pyrazole compound [3-(3,5-dimethyl-1H-pyrazol-1-yl)benzyl]amine, and (c) Fmoc-protected valine with diphenyl amine. Conductor-like screening model for real solvents (COSMO-RS) was used to obtain sigma profiles of the hybrid mixtures and to predict viscosities and mixing enthalpies of each composite. These results were used to determine optimal ionic liquid-bio-organic amphiphile mixtures. Molecular dynamics simulations of three optimal hybrids were then performed, and the interactions involved in the formation of the hybrids were analyzed. Our results indicated that cholinium-based ILs interacted most favorably with the amphiphiles through a variety of inter- and intramolecular interactions. This work serves to illustrate important factors that influence the interactions between bio-organic amphiphiles and bio-ILs and aids in the development of novel ionic liquid-based composites for a wide variety of potential biological applications.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Benedetto A.; Ballone P. Room Temperature Ionic Liquids Meet Biomolecules: A Microscopic View of Structure and Dynamics. ACS Sustainable Chem. Eng. 2016, 4, 392–412. 10.1021/acssuschemeng.5b01385. - DOI
-
- Keaveney S. T.; Harper J. B.; Croft A. K. Computational Approaches to Understanding Reaction Outcomes of Organic Processes in Ionic Liquids. RSC Adv. 2015, 5, 35709–35729. 10.1039/C4RA14676J. - DOI
LinkOut - more resources
Full Text Sources

