Fabrication of GQD-Electrodeposited Screen-Printed Carbon Electrodes for the Detection of the CRP Biomarker
- PMID: 34901602
- PMCID: PMC8655768
- DOI: 10.1021/acsomega.1c04043
Fabrication of GQD-Electrodeposited Screen-Printed Carbon Electrodes for the Detection of the CRP Biomarker
Abstract
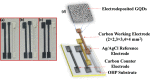
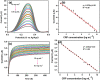
The traditional three-electrode electrochemical system used in the development of biosensors for detecting various biomarkers of interest necessitates the use of bulk electrodes, which precludes the deployment of handheld electrochemical devices in clinical applications. Affordable screen-printed carbon electrodes (SPCEs) modified with functional interfaces are being developed to enhance the sensitivity of a compact sensing system as a whole. In this work, SPCEs were fabricated on an overhead projection (OHP) sheet in three different active areas of 2 × 2, 3 × 3, and 4 × 4 mm2 using a screen printing technique, and then ∼2 nm sized graphene quantum dots (GQDs) were electrodeposited over the SPCE surface to add functionality for the detection of ultralow levels of one of the cardiac biomarkers, C-reactive protein (CRP). The proposed mediator-dependent voltammetric biosensor exhibited good sensitivity, a low detection limit, and a linear range of 2.45 μA ng-1 mL-1 cm-2, 0.036 ng mL-1, and 0.5-10 ng mL-1, respectively. The fabricated SPCE/GQDs/anti-CRP biosensor could rapidly detect CRP in less than 25 s. The intra- and interassays were performed with five sensor strips, which showed a minimum standard deviation of 1.85 and 2.8%, respectively. The SPCE/GQDs/anti-CRP electrode was used to detect CRP concentrations in a ringer lactate solution. Thus, the developed biosensor has all of the characteristics such as rapidity, inexpensive disposable electrodes, miniaturization, and a lower detection limit needed to evolve as a point-of-care (PoC) application.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Pepys M. B.; Hirschfield G. M.; Tennent G. A.; Gallimore J. R.; Kahan M. C.; Bellotti V.; Hawkins P. N.; Myers R. M.; Smith M. D.; Polara A.; Cobb A. J. A.; Ley S. V.; Aquilina J. A.; Robinson C. V.; Sharif I.; Gray G. A.; Sabin C. A.; Jenvey M. C.; Kolstoe S. E.; Thompson D.; Wood S. P. Targeting C-Reactive Protein for the Treatment of Cardiovascular Disease. Nature 2006, 440, 1217–1221. 10.1038/nature04672. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

