Intravitreal bevacizumab prior to vitrectomy for proliferative diabetic retinopathy: a systematic review
- PMID: 34901749
- PMCID: PMC8655445
- DOI: 10.1177/25158414211059256
Intravitreal bevacizumab prior to vitrectomy for proliferative diabetic retinopathy: a systematic review
Abstract
Background: Diabetic retinopathy is a leading cause of visual loss in the working population. Pars plana vitrectomy has become the mainstream treatment option for severe proliferative diabetic retinopathy (PDR) associated with significant vitreous haemorrhage and/or tractional retinal detachment. Despite the advances in surgical equipment, diabetic vitrectomy remains a challenging operation, requiring advanced microsurgical skills, especially in the presence of tractional retinal detachment. Preoperative intravitreal bevacizumab has been widely employed as an adjuvant to ease surgical difficulty and improve postoperative prognosis.Aims: This study aims to assess the effectiveness of preoperative intravitreal bevacizumab in reducing intraoperative complications and improving postoperative outcomes in patients undergoing vitrectomy for the complications of PDR.
Methods: A literature search was conducted using the PubMed, Cochrane, and ClinicalTrials.gov databases to identify all related studies published before 31/10/2020. Prespecified outcome measures were operation time, intraoperative iatrogenic retinal breaks, best-corrected visual acuity in the last follow-up visit, the presence of any postoperative vitreous haemorrhage and the need to re-operate. Evidence synthesis was performed using Fixed or Random Effects models, depending on the heterogeneity of the included studies. Heterogeneity was assessed using Q-statistic and I2. Additional meta-regression models, subgroup analyses and sensitivity analyses were performed as appropriate.
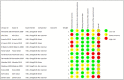
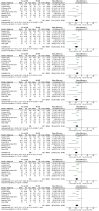
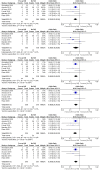
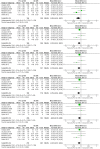
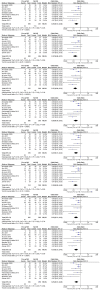
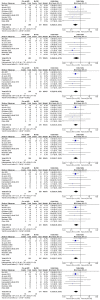
Results: Thirteen randomized control trials, with a total of 688 eyes were included in this review. Comparison of the intraoperative data showed that bevacizumab reduced operation time (p < 0.001), minimized iatrogenic retinal breaks (p < 0.001), provided better long-term visual acuity outcomes (p = 0.005), and prevented vitreous haemorrhage (p < 0.001) and the need for reoperation (p = 0.001 < 0.05). Findings were strongly corroborated by additional sensitivity and subgroup analyses.
Conclusion: Preoperative administration of bevacizumab is effective in reducing intraoperative complications and improving the postoperative prognosis of diabetic vitrectomy.PROSPERO registration number: CRD42021219280.
Keywords: bevacizumab; diabetic retinopathy; systematic review; vitrectomy.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Professor David Steel (all unrelated to current work): Alcon – Grant funding, consultancy Roche – Consultancy Bayer – Grant funding Gyroscope – Consultancy Novartis – Consultancy
Figures

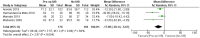
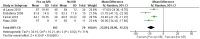

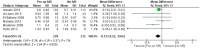









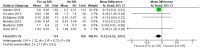


Similar articles
-
Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy.Graefes Arch Clin Exp Ophthalmol. 2010 Jun;248(6):785-91. doi: 10.1007/s00417-010-1303-3. Epub 2010 Feb 5. Graefes Arch Clin Exp Ophthalmol. 2010. PMID: 20135139 Clinical Trial.
-
Early vitreous hemorrhage after vitrectomy with preoperative intravitreal bevacizumab for proliferative diabetic retinopathy.Middle East Afr J Ophthalmol. 2013 Jan-Mar;20(1):51-5. doi: 10.4103/0974-9233.106387. Middle East Afr J Ophthalmol. 2013. PMID: 23580852 Free PMC article.
-
Preoperative Bevacizumab for Tractional Retinal Detachment in Proliferative Diabetic Retinopathy: A Prospective Randomized Clinical Trial.Am J Ophthalmol. 2019 Nov;207:279-287. doi: 10.1016/j.ajo.2019.05.007. Epub 2019 May 13. Am J Ophthalmol. 2019. PMID: 31095954 Clinical Trial.
-
Preoperative intravitreal bevacizumab for proliferative diabetic retinopathy patients undergoing vitrectomy - First update.Medwave. 2019 Jan 25;19(1):e7512. doi: 10.5867/medwave.2019.01.7511. Medwave. 2019. PMID: 30816881 Review. English, Spanish.
-
Network meta-analysis of intravitreal conbercept as an adjuvant to vitrectomy for proliferative diabetic retinopathy.Front Endocrinol (Lausanne). 2023 Feb 22;14:1098165. doi: 10.3389/fendo.2023.1098165. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36896181 Free PMC article. Review.
Cited by
-
Comment on - Evaluation of 27-gauge vitrectomy for complex proliferative diabetic retinopathy.Oman J Ophthalmol. 2024 Jun 27;17(2):308. doi: 10.4103/ojo.ojo_141_23. eCollection 2024 May-Aug. Oman J Ophthalmol. 2024. PMID: 39132120 Free PMC article. No abstract available.
-
Prophylactic intravitreal injection of aflibercept for preventing postvitrectomy hemorrhage in proliferative diabetic retinopathy: A randomized controlled trial.Front Public Health. 2023 Jan 11;10:1067670. doi: 10.3389/fpubh.2022.1067670. eCollection 2022. Front Public Health. 2023. PMID: 36711366 Free PMC article. Clinical Trial.
-
Anti-vascular endothelial growth factors in combination with vitrectomy for complications of proliferative diabetic retinopathy.Cochrane Database Syst Rev. 2023 May 31;5(5):CD008214. doi: 10.1002/14651858.CD008214.pub4. Cochrane Database Syst Rev. 2023. PMID: 37260074 Free PMC article. Review.
References
-
- Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology 1978; 85: 82–106. - PubMed
-
- Lincoff H, Serag Y, Chang S, et al.. Tractional elevations of the retina in patients with diabetes. Am J Ophthalmol 1992; 113: 235–242. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

