Electrostatic flocking of salt-treated microfibers and nanofiber yarns for regenerative engineering
- PMID: 34901819
- PMCID: PMC8640530
- DOI: 10.1016/j.mtbio.2021.100166
Electrostatic flocking of salt-treated microfibers and nanofiber yarns for regenerative engineering
Abstract
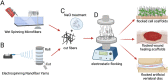
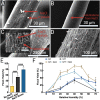
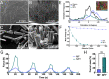
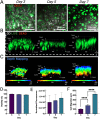
Electrostatic flocking is a textile technology that employs a Coulombic driving force to launch short fibers from a charging source towards an adhesive-covered substrate, resulting in a dense array of aligned fibers perpendicular to the substrate. However, electrostatic flocking of insulative polymeric fibers remains a challenge due to their insufficient charge accumulation. We report a facile method to flock electrostatically insulative poly(ε-caprolactone) (PCL) microfibers (MFs) and electrospun PCL nanofiber yarns (NFYs) by incorporating NaCl during pre-flock processing. Both MF and NFY were evaluated for flock functionality, mechanical properties, and biological responses. To demonstrate this platform's diverse applications, standalone flocked NFY and MF scaffolds were synthesized and evaluated as scaffold for cell growth. Employing the same methodology, scaffolds made from poly(glycolide-co-l-lactide) (PGLA) (90:10) MFs were evaluated for their wound healing capacity in a diabetic mouse model. Further, a flock-reinforced polydimethylsiloxane (PDMS) disc was fabricated to create an anisotropic artificial vertebral disc (AVD) replacement potentially used as a treatment for lumbar degenerative disc disease. Overall, a salt-based flocking method is described with MFs and NFYs, with wound healing and AVD repair applications presented.
Keywords: Artificial vertebral disc; Electrostatic flocking; Microfibers; Nanofiber yarns; Wound healing.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Fang Y., Xu Y., Wang Z., Zhou W., Yan L., Fan X., Liu H. 3D porous chitin sponge with high absorbency, rapid shape recovery, and excellent antibacterial activities for noncompressible wound. Chem. Eng. J. 2020;388:124169. doi: 10.1016/j.cej.2020.124169. - DOI
-
- John J.V., McCarthy A., Wang H., Luo Z., Li H., Wang Z., Cheng F., Zhang Y.S., Xie J. Freeze-casting with 3D-printed templates creates anisotropic microchannels and patterned macrochannels within biomimetic nanofiber aerogels for rapid cellular infiltration. Adv. Healthc. Mater. 2021;12:2100238. doi: 10.1002/adhm.202100238. - DOI - PMC - PubMed
-
- Balasubramanian P., Boccaccini A.R. Bilayered bioactive glass scaffolds incorporating fibrous morphology by flock technology. Mater. Lett. 2015;158:313–316. doi: 10.1016/j.matlet.2015.06.036. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

