Long-term real-world effectiveness of allergy immunotherapy in patients with allergic rhinitis and asthma: Results from the REACT study, a retrospective cohort study
- PMID: 34901915
- PMCID: PMC8640513
- DOI: 10.1016/j.lanepe.2021.100275
Long-term real-world effectiveness of allergy immunotherapy in patients with allergic rhinitis and asthma: Results from the REACT study, a retrospective cohort study
Abstract
Background: Allergen immunotherapy (AIT) is the only causal treatment for respiratory allergy. Long-term real-life effectiveness of AIT remains to be demonstrated beyond the evidence from randomised controlled trials (RCTs).
Methods: REACT (Real world effectiveness in allergy immunotherapy) is a retrospective cohort study using claims data between 2007 and 2017. Study eligibility was a confirmed diagnosis of allergic rhinitis (AR), with or without asthma, and AIT. To ensure comparable groups, AIT-treated subjects were propensity score matched 1:1 with control subjects, using characteristic and potential confounding variables. Outcomes were analysed as within (pre vs post AIT) and between (AIT vs control) group differences across 9 years of follow-up (ClinicalTrial.gov: NCT04125888).
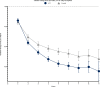
Findings: 46,024 AIT-treated subjects were matched with control subjects and 14,614 were included in the pre-existing asthma cohort. AIT-treated subjects were 29·5 (16·3) years and 53% were male. Compared to pre-index year, AIT was consistently associated with greater reductions compared to control subjects in AR and asthma prescriptions, including both asthma controller and reliever prescriptions. Additionally, the AIT group had significantly greater likelihood of stepping down asthma treatment (P <0·0001). In addition to the reduction in asthma treatment in the AIT group, a greater reduction in severe asthma exacerbations was demonstrated (P<0·05). Reductions in pneumonia with antibiotic prescriptions, hospitalisations, and duration of inpatients stays were all in favour of AIT.
Interpretation: The study extends the existing RCT evidence for AIT by demonstrating longer-term and sustained effectiveness of AIT in the real world. Additionally, in patients with concurrent asthma, AIT was associated with reduced likelihood of asthma exacerbations and pneumonia.
Funding: The study was funded by ALK A/S.
Keywords: AIT, allergy immunotherapy; AR, allergic rhinitis; Allergic rhinitis; Allergy; Allergy immunotherapy; Asthma; Effectiveness; FU, follow-up; HDM, house dust mite; HRU, health care resource utilisation; ICS, inhaled corticosteroids; INCS, intranasal corticosteroids; LABA, long-acting beta2-agonists; PSM, propensity score matching; RCT, randomised clinical trial; RWE, real world evidence; Real-world evidence; Retrospective cohort study; Rx, prescription; SABA, short-acting beta2-agonists; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy.
© 2021 The Authors.
Conflict of interest statement
Dr. Fritzsching reports personal fees (pertaining travelling to study meeting) from ALK during the conduct of the study; and speaker honorarium from Novartis and from Merck Sharp & Dohme, outside the submitted work. Dr. Contoli reports personal fees from Alk-Abello, during the conduct of the study; grants, personal fees and non-financial support from Chiesi, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, grants, personal fees and non-financial support from GlaxoSmithKline, personal fees and non-financial support from Novartis, personal fees and non-financial support from Zambon, grants from University of Ferrara - Italy, outside the submitted work. Dr. Porsbjerg reports grants from ALK, grants and personal fees from Astra Zeneca, grants and personal fees from GSK, grants and personal fees from Novartis, grants, and personal fees from Chiesi, grants and personal fees from Sanofi, grants and personal fees from TEVA, outside the submitted work. Sarah Buchs reports to be employed at ALK-Abello. Dr. Rask Larsen reports being an employee at ALK. Dr. Elliott has nothing to disclose. Ms. Romano Rodriguez reports she is an ALK employee. Dr. Freemantle reports personal fees from Astrazeneca, personal fees from Ipsen, personal fees from Sanofi Aventis, personal fees from Grifols, personal fees from Novatis, personal fees from Aimmune, personal fees from Vertex, personal fees from MSD, personal fees from Allergan, outside the submitted work.
Figures


References
-
- Pawankar R.C., Holgate S.T., Lockey R.F., et al. Wisconsin World Allergy Organization; Milwaukee: 2013. WAO white book on allergy 2013 update. GW.
-
- Bousquet J., Hellings P.W., Agache I., et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Phase 4 (2018): change management in allergic rhinitis and asthma multimorbidity using mobile technology. J Allergy Clin Immunol. 2019;143(3):864–879. - PubMed
-
- GINA Global Strategy for Ashtma Management and Prevention. 2021
-
- Bousquet J., Van Cauwenberge P., Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5):S147–S334. Suppl. - PubMed
-
- Braido F., Baiardini I., Brandi S., Porcu A., Canonica G.W. Allergic rhinitis and asthma ad hoc survey: clinical and psychological perspectives. Clin Exp Allergy. 2007;37(5):788–793. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

