Efficacy and Safety of COVID-19 Convalescent Plasma in Hospitalized Patients: A Randomized Clinical Trial
- PMID: 34901997
- PMCID: PMC8669605
- DOI: 10.1001/jamainternmed.2021.6850
Efficacy and Safety of COVID-19 Convalescent Plasma in Hospitalized Patients: A Randomized Clinical Trial
Abstract
Importance: There is clinical equipoise for COVID-19 convalescent plasma (CCP) use in patients hospitalized with COVID-19.
Objective: To determine the safety and efficacy of CCP compared with placebo in hospitalized patients with COVID-19 receiving noninvasive supplemental oxygen.
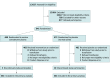
Design, setting, and participants: CONTAIN COVID-19, a randomized, double-blind, placebo-controlled trial of CCP in hospitalized adults with COVID-19, was conducted at 21 US hospitals from April 17, 2020, to March 15, 2021. The trial enrolled 941 participants who were hospitalized for 3 or less days or presented 7 or less days after symptom onset and required noninvasive oxygen supplementation.
Interventions: A unit of approximately 250 mL of CCP or equivalent volume of placebo (normal saline).
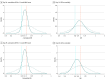
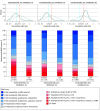
Main outcomes and measures: The primary outcome was participant scores on the 11-point World Health Organization (WHO) Ordinal Scale for Clinical Improvement on day 14 after randomization; the secondary outcome was WHO scores determined on day 28. Subgroups were analyzed with respect to age, baseline WHO score, concomitant medications, symptom duration, CCP SARS-CoV-2 titer, baseline SARS-CoV-2 serostatus, and enrollment quarter. Outcomes were analyzed using a bayesian proportional cumulative odds model. Efficacy of CCP was defined as a cumulative adjusted odds ratio (cOR) less than 1 and a clinically meaningful effect as cOR less than 0.8.
Results: Of 941 participants randomized (473 to placebo and 468 to CCP), 556 were men (59.1%); median age was 63 years (IQR, 52-73); 373 (39.6%) were Hispanic and 132 (14.0%) were non-Hispanic Black. The cOR for the primary outcome adjusted for site, baseline risk, WHO score, age, sex, and symptom duration was 0.94 (95% credible interval [CrI], 0.75-1.18) with posterior probability (P[cOR<1] = 72%); the cOR for the secondary adjusted outcome was 0.92 (95% CrI, 0.74-1.16; P[cOR<1] = 76%). Exploratory subgroup analyses suggested heterogeneity of treatment effect: at day 28, cORs were 0.72 (95% CrI, 0.46-1.13; P[cOR<1] = 93%) for participants enrolled in April-June 2020 and 0.65 (95% CrI, 0.41 to 1.02; P[cOR<1] = 97%) for those not receiving remdesivir and not receiving corticosteroids at randomization. Median CCP SARS-CoV-2 neutralizing titer used in April to June 2020 was 1:175 (IQR, 76-379). Any adverse events (excluding transfusion reactions) were reported for 39 (8.2%) placebo recipients and 44 (9.4%) CCP recipients (P = .57). Transfusion reactions occurred in 2 (0.4) placebo recipients and 8 (1.7) CCP recipients (P = .06).
Conclusions and relevance: In this trial, CCP did not meet the prespecified primary and secondary outcomes for CCP efficacy. However, high-titer CCP may have benefited participants early in the pandemic when remdesivir and corticosteroids were not in use.
Trial registration: ClinicalTrials.gov Identifier: NCT04364737.
Conflict of interest statement
Figures

References
-
- Kraft CS, Hewlett AL, Koepsell S, et al. ; Nebraska Biocontainment Unit and the Emory Serious Communicable Diseases Unit . The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clin Infect Dis. 2015;61(4):496-502. doi:10.1093/cid/civ334 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

