Inhaled Corticosteroids Alone and in Combination With Long-Acting β2 Receptor Agonists to Treat Reduced Lung Function in Preterm-Born Children: A Randomized Clinical Trial
- PMID: 34902004
- PMCID: PMC8669602
- DOI: 10.1001/jamapediatrics.2021.5111
Inhaled Corticosteroids Alone and in Combination With Long-Acting β2 Receptor Agonists to Treat Reduced Lung Function in Preterm-Born Children: A Randomized Clinical Trial
Erratum in
-
Labeling Errors in Flow Diagram.JAMA Pediatr. 2023 Feb 1;177(2):213. doi: 10.1001/jamapediatrics.2022.5481. JAMA Pediatr. 2023. PMID: 36574253 Free PMC article. No abstract available.
Abstract
Importance: Decreases in future lung function are a hallmark of preterm birth, but studies for management of decreased lung function are limited.
Objective: To determine whether 12 weeks of treatment with inhaled corticosteroids (ICS) alone or in combination with long-acting β2 agonists (LABA) improves spirometry and exercise capacity in school-aged preterm-born children who had percent predicted forced expiratory volume in 1 second (%FEV1) less than or equal to 85% compared with inhaled placebo treatment.
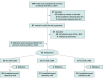
Design, setting, and participants: A double-blind, randomized, placebo-controlled trial was conducted to evaluate ICS and ICS/LABA against placebo. Preterm-born children (age, 7-12 years; gestation ≤34 weeks at birth) who did not have clinically significant congenital, cardiopulmonary, or neurodevelopmental abnormalities underwent spirometry, exercise testing, and measurement of fractional exhaled nitric oxide before and after treatment. A total of 144 preterm-born children at the Children's Hospital for Wales in Cardiff, UK, were identified and enrolled between July 1, 2017, and August 31, 2019.
Interventions: Each child was randomized to 1 of 3 cohorts: fluticasone propionate, 50 μg, with placebo; fluticasone propionate, 50 μg, with salmeterol, 25 μg; or placebo inhalers, all given as 2 puffs twice daily for 12 weeks. Children receiving preexisting ICS treatment underwent washout prior to randomization to ICS or ICS/LABA.
Main outcomes and measures: The primary outcome was between-group differences assessed by adjusted pretreatment and posttreatment differences of %FEV1 using analysis of covariance. Intention-to-treat analysis was conducted.
Results: Of 144 preterm-born children who were identified with %FEV1 less than or equal to 85%, 53 were randomized. Treatment allocation was 20 children receiving ICS (including 5 with prerandomization ICS), 19 children receiving ICS/LABA (including 4 with prerandomization ICS), and 14 children receiving placebo. The mean (SD) age of children was 10.8 (1.2) years, and 29 of the randomized children (55%) were female. The posttreatment %FEV1 was adjusted for sex, gestation, bronchopulmonary dysplasia, intrauterine growth restriction, pretreatment corticosteroid status, treatment group, and pretreatment values. Posttreatment adjusted means for %FEV1, using analysis of covariance, were 7.7% (95% CI, -0.27% to 15.72%; P = .16) higher in the ICS group and 14.1% (95% CI, 7.3% to 21.0%; P = .002) higher in the ICS/LABA group compared with the placebo group. Active treatment decreased the fractional exhaled nitric oxide and improved postexercise bronchodilator response but did not improve exercise capacity. One child developed cough when starting inhaler treatment; no other adverse events reported during the trial could be attributed to the inhaler treatment.
Conclusions and relevance: The results of this randomized clinical trial suggest that combined ICS/LABA treatment is beneficial for prematurity-associated lung disease in children.
Trial registration: EudraCT number: 2015-003712-20.
Conflict of interest statement
Figures

Comment in
-
ICS/LABA für ehemalige Frühgeborene?MMW Fortschr Med. 2022 Feb;164(3):31. doi: 10.1007/s15006-022-0801-7. MMW Fortschr Med. 2022. PMID: 35146664 Review. German. No abstract available.
-
Inhaled Corticosteroids and Long-Acting β2 Receptor Agonists for Preterm-Born Children-New Insights but Still Many Questions-Reply.JAMA Pediatr. 2022 Jun 1;176(6):615-616. doi: 10.1001/jamapediatrics.2022.0300. JAMA Pediatr. 2022. PMID: 35377402 No abstract available.
-
Inhaled Corticosteroids and Long-Acting β2 Receptor Agonists for Preterm-Born Children-New Insights but Still Many Questions.JAMA Pediatr. 2022 Jun 1;176(6):614-615. doi: 10.1001/jamapediatrics.2022.0303. JAMA Pediatr. 2022. PMID: 35377410 No abstract available.
References
-
- Doyle LW, Andersson S, Bush A, et al. ; Adults born Preterm International Collaboration . Expiratory airflow in late adolescence and early adulthood in individuals born very preterm or with very low birthweight compared with controls born at term or with normal birthweight: a meta-analysis of individual participant data. Lancet Respir Med. 2019;7(8):677-686. doi: 10.1016/S2213-2600(18)30530-7 - DOI - PubMed

