Association Between Antiseizure Drug Monotherapy and Mortality for Patients With Poststroke Epilepsy
- PMID: 34902006
- PMCID: PMC8669603
- DOI: 10.1001/jamaneurol.2021.4584
Association Between Antiseizure Drug Monotherapy and Mortality for Patients With Poststroke Epilepsy
Abstract
Importance: There is little evidence to guide the choice of antiseizure medication (ASM) for patients with poststroke epilepsy. Theoretical concerns about detrimental effects of ASMs on survival exist. Enzyme-inducing drugs could interfere with secondary stroke prevention. The US Food and Drug Administration recently issued a safety announcement about the potential proarrhythmic properties of lamotrigine.
Objective: To investigate whether mortality varies with specific ASMs among patients with poststroke epilepsy.
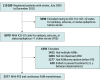
Design, setting, and participants: A cohort study was conducted using individual-level data from linked registers on all adults in Sweden with acute stroke from July 1, 2005, to December 31, 2010, and subsequent onset of epilepsy before December 31, 2014. A total of 2577 patients receiving continuous ASM monotherapy were eligible for the study. Data were analyzed between May 27, 2019, and April 8, 2021.
Exposures: The dispensed ASM (Anatomical Therapeutic Chemical code N03A) determined exposure status, and the first dispensation date marked the start of treatment.
Main outcomes and measures: The primary outcome, all-cause death, was analyzed using Cox proportional hazards regression with carbamazepine as the reference. Cardiovascular death (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes I0-I99 as the underlying cause) was assessed using Fine-Gray competing risk regression models.
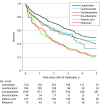
Results: A total of 2577 patients (1400 men [54%]; median age, 78 years [IQR, 69-85 years]) were included. The adjusted hazard ratio of all-cause death compared with carbamazepine was 0.72 (95% CI, 0.60-0.86) for lamotrigine, 0.96 (95% CI, 0.80-1.15) for levetiracetam, 1.40 (95% CI, 1.23-1.59) for valproic acid, 1.16 (95% CI, 0.88-1.51) for phenytoin, and 1.16 (95% CI, 0.81-1.66) for oxcarbazepine. The adjusted hazard ratio of cardiovascular death compared with carbamazepine was 0.76 (95% CI, 0.61-0.95) for lamotrigine, 0.77 (95% CI, 0.60-0.99) for levetiracetam, 1.40 (95% CI, 1.19-1.64) for valproic acid, 1.02 (95% CI, 0.71-1.47) for phenytoin, and 0.71 (95% CI, 0.42-1.18) for oxcarbazepine.
Conclusions and relevance: This cohort study's findings suggest differences in survival between patients treated with different ASMs for poststroke epilepsy. Patients receiving lamotrigine monotherapy had significantly lower mortality compared with those receiving carbamazepine. The opposite applied to patients prescribed valproic acid, who had a higher risk of cardiovascular and all-cause death. Levetiracetam was associated with a reduced risk of cardiovascular death compared with carbamazepine, but there was no significant difference in overall mortality.
Conflict of interest statement
Figures
Similar articles
-
Retention rate of first antiepileptic drug in poststroke epilepsy: A nationwide study.Seizure. 2019 Jan;64:29-33. doi: 10.1016/j.seizure.2018.11.013. Epub 2018 Nov 23. Seizure. 2019. PMID: 30529757
-
Risk of Major Congenital Malformations and Exposure to Antiseizure Medication Monotherapy.JAMA Neurol. 2024 May 1;81(5):481-489. doi: 10.1001/jamaneurol.2024.0258. JAMA Neurol. 2024. PMID: 38497990 Free PMC article.
-
Association of antiseizure medications and adverse cardiovascular events: A global health federated network analysis.Epilepsia. 2024 May;65(5):1264-1274. doi: 10.1111/epi.17922. Epub 2024 Feb 27. Epilepsia. 2024. PMID: 38411304
-
Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2019 Jul 18;7(7):CD001911. doi: 10.1002/14651858.CD001911.pub4. Cochrane Database Syst Rev. 2019. PMID: 31318037 Free PMC article.
-
Breastfeeding while on treatment with antiseizure medications: a systematic review from the ILAE Women Task Force.Epileptic Disord. 2022 Dec 1;24(6):1020-1032. doi: 10.1684/epd.2022.1492. Epileptic Disord. 2022. PMID: 36193017 English.
Cited by
-
Further advances in epilepsy.J Neurol. 2023 Nov;270(11):5655-5670. doi: 10.1007/s00415-023-11860-6. Epub 2023 Jul 17. J Neurol. 2023. PMID: 37458794 Review.
-
St3gal5-mediated sialylation of glyco-CD177 on neutrophils restricts neuroinflammation following CNS injury.Proc Natl Acad Sci U S A. 2025 Apr 22;122(16):e2426187122. doi: 10.1073/pnas.2426187122. Epub 2025 Apr 17. Proc Natl Acad Sci U S A. 2025. PMID: 40244680
-
Epilepsy in Cerebrovascular Diseases: A Narrative Review.Curr Neuropharmacol. 2023;21(8):1634-1645. doi: 10.2174/1570159X20666220706113925. Curr Neuropharmacol. 2023. PMID: 35794769 Free PMC article. Review.
-
Exit Strategy: Balancing the Risks and Rewards of Antiseizure Medication Withdrawal.Epilepsy Curr. 2024 Mar 25;24(3):150-155. doi: 10.1177/15357597241238898. eCollection 2024 May-Jun. Epilepsy Curr. 2024. PMID: 38898899 Free PMC article. Review.
-
Chinese Stroke Association guidelines for clinical management of ischaemic cerebrovascular diseases: executive summary and 2023 update.Stroke Vasc Neurol. 2023 Dec 29;8(6):e3. doi: 10.1136/svn-2023-002998. Stroke Vasc Neurol. 2023. PMID: 38158224 Free PMC article.
References
-
- Arntz RM, Rutten-Jacobs LC, Maaijwee NA, et al. . Poststroke epilepsy is associated with a high mortality after a stroke at young age: follow-up of Transient Ischemic Attack and Stroke Patients and Unelucidated Risk Factor Evaluation study. Stroke. 2015;46(8):2309-2311. doi:10.1161/STROKEAHA.115.010115 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

