Performance of a Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography-Derived Risk-Stratification Tool for High-risk and Very High-risk Prostate Cancer
- PMID: 34902034
- PMCID: PMC8669522
- DOI: 10.1001/jamanetworkopen.2021.38550
Performance of a Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography-Derived Risk-Stratification Tool for High-risk and Very High-risk Prostate Cancer
Abstract
Importance: Prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) can detect low-volume, nonlocalized (ie, regional or metastatic) prostate cancer that was occult on conventional imaging. However, the long-term clinical implications of PSMA PET/CT upstaging remain unclear.
Objectives: To evaluate the prognostic significance of a nomogram that models an individual's risk of nonlocalized upstaging on PSMA PET/CT and to compare its performance with existing risk-stratification tools.
Design, setting, and participants: This cohort study included patients diagnosed with high-risk or very high-risk prostate cancer (ie, prostate-specific antigen [PSA] level >20 ng/mL, Gleason score 8-10, and/or clinical stage T3-T4, without evidence of nodal or metastatic disease by conventional workup) from April 1995 to August 2018. This multinational study was conducted at 15 centers. Data were analyzed from December 2020 to March 2021.
Exposures: Curative-intent radical prostatectomy (RP), external beam radiotherapy (EBRT), or EBRT plus brachytherapy (BT), with or without androgen deprivation therapy.
Main outcomes and measures: PSMA upstage probability was calculated from a nomogram using the biopsy Gleason score, percentage positive systematic biopsy cores, clinical T category, and PSA level. Biochemical recurrence (BCR), distant metastasis (DM), prostate cancer-specific mortality (PCSM), and overall survival (OS) were analyzed using Fine-Gray and Cox regressions. Model performance was quantified with the concordance (C) index.
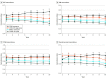
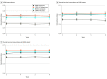
Results: Of 5275 patients, the median (IQR) age was 66 (60-72) years; 2883 (55%) were treated with RP, 1669 (32%) with EBRT, and 723 (14%) with EBRT plus BT; median (IQR) PSA level was 10.5 (5.9-23.2) ng/mL; 3987 (76%) had Gleason grade 8 to 10 disease; and 750 (14%) had stage T3 to T4 disease. Median (IQR) follow-up was 5.1 (3.1-7.9) years; 1221 (23%) were followed up for at least 8 years. Overall, 1895 (36%) had BCR, 851 (16%) developed DM, and 242 (5%) died of prostate cancer. PSMA upstage probability was significantly prognostic of all clinical end points, with 8-year C indices of 0.63 (95% CI, 0.61-0.65) for BCR, 0.69 (95% CI, 0.66-0.71) for DM, 0.71 (95% CI, 0.67-0.75) for PCSM, and 0.60 (95% CI, 0.57-0.62) for PCSM (P < .001). The PSMA nomogram outperformed existing risk-stratification tools, except for similar performance to Staging Collaboration for Cancer of the Prostate (STAR-CAP) for PCSM (eg, DM: PSMA, 0.69 [95% CI, 0.66-0.71] vs STAR-CAP, 0.65 [95% CI, 0.62-0.68]; P < .001; Memorial Sloan Kettering Cancer Center nomogram, 0.57 [95% CI, 0.54-0.60]; P < .001; Cancer of the Prostate Risk Assessment groups, 0.53 [95% CI, 0.51-0.56]; P < .001). Results were validated in secondary cohorts from the Surveillance, Epidemiology, and End Results database and the National Cancer Database.
Conclusions and relevance: These findings suggest that PSMA upstage probability is associated with long-term, clinically meaningful end points. Furthermore, PSMA upstaging had superior risk discrimination compared with existing tools. Formerly occult, PSMA PET/CT-detectable nonlocalized disease may be the main driver of outcomes in high-risk patients.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

