Diabetic macular ischaemia- a new therapeutic target?
- PMID: 34902545
- PMCID: PMC11268431
- DOI: 10.1016/j.preteyeres.2021.101033
Diabetic macular ischaemia- a new therapeutic target?
Abstract
Diabetic macular ischaemia (DMI) is traditionally defined and graded based on the angiographic evidence of an enlarged and irregular foveal avascular zone. However, these anatomical changes are not surrogate markers for visual impairment. We postulate that there are vascular phenotypes of DMI based on the relative perfusion deficits of various retinal capillary plexuses and choriocapillaris. This review highlights several mechanistic pathways, including the role of hypoxia and the complex relation between neurons, glia, and microvasculature. The current animal models are reviewed, with shortcomings noted. Therefore, utilising the advancing technology of optical coherence tomography angiography (OCTA) to identify the reversible DMI phenotypes may be the key to successful therapeutic interventions for DMI. However, there is a need to standardise the nomenclature of OCTA perfusion status. Visual acuity is not an ideal endpoint for DMI clinical trials. New trial endpoints that represent disease progression need to be developed before irreversible vision loss in patients with DMI. Natural history studies are required to determine the course of each vascular and neuronal parameter to define the DMI phenotypes. These DMI phenotypes may also partly explain the development and recurrence of diabetic macular oedema. It is also currently unclear where and how DMI fits into the diabetic retinopathy severity scales, further highlighting the need to better define the progression of diabetic retinopathy and DMI based on both multimodal imaging and visual function. Finally, we discuss a complete set of proposed therapeutic pathways for DMI, including cell-based therapies that may provide restorative potential.
Keywords: Diabetic macular ischaemia; Diabetic macular oedema; Diabetic retinopathy; Foveal avascular zone; Optical coherence tomography; Optical coherence tomography angiography.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declarations of interest
Chui Ming Gemmy Cheung: Bayer, Novartis, Roche, Allergan, Boehringer-Ingelheim, Topcon, Zeiss.
Amani Fawzi: Roche, Regeneron, Genentech, Boehringer-Ingelheim.
Kelvin YC Teo: Bayer, Novartis, Roche.
Sagnik Sen: None.
Hisashi Fukuyama: None.
Wei-Shan Tsai: None.
Sobha Sivaprasad has received funding/fees from Bayer, Novartis, Allergan, Roche, Boehringer Ingelheim, Optos, Oxurion, Oculis, Biogen, Apellis and Heidelberg Engineering.
Figures



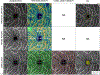








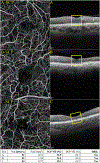

References
-
- Diabetic Retinopathy Clinical Research, N., Elman MJ., Aiello LP., Beck RW., Bressler NM., Bressler SB., Edwards AR., Ferris FL 3rd., Friedman SM., Glassman AR., Miller KM., Scott IU., Stockdale CR., Sun JK, 2010. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 117, 1064–1077 e1035. - PMC - PubMed
-
- Agemy SA, Scripsema NK, Shah CM, Chui T, Garcia PM, Lee JG, Gentile RC, Hsiao Y-S, Zhou Q, Ko T, 2015. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina 35, 2353–2363. - PubMed
-
- Aiello LP, Cahill MT, Wong JS, 2001. Systemic considerations in the management of diabetic retinopathy. Am. J. Ophthalmol. 132, 760–776. - PubMed
-
- Al-Sheikh M, Akil H, Pfau M, Sadda SR, 2016. Swept-source OCT angiography imaging of the foveal avascular zone and macular capillary network density in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 57, 3907–3913. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

