Lentiviral Infections Persist in Brain despite Effective Antiretroviral Therapy and Neuroimmune Activation
- PMID: 34903055
- PMCID: PMC8669467
- DOI: 10.1128/mBio.02784-21
Lentiviral Infections Persist in Brain despite Effective Antiretroviral Therapy and Neuroimmune Activation
Abstract
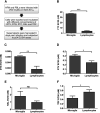
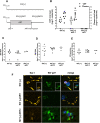
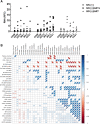
HIV infection persists in different tissue reservoirs among people with HIV (PWH) despite effective antiretroviral therapy (ART). In the brain, lentiviruses replicate principally in microglia and trafficking macrophages. The impact of ART on this viral reservoir is unknown. We investigated the activity of contemporary ART in various models of lentivirus brain infection. HIV-1 RNA and total and integrated DNA were detected in cerebral cortex from all PWH (n = 15), regardless of ART duration or concurrent plasma viral quantity and, interestingly, integrated proviral DNA levels in brain were significantly higher in the aviremic ART-treated group (P < 0.005). Most ART drugs tested (dolutegravir, ritonavir, raltegravir, and emtricitabine) displayed significantly lower 50% effective concentration (EC50) values in lymphocytes than in microglia, except tenofovir, which showed 1.5-fold greater activity in microglia (P < 0.05). In SIV-infected Chinese rhesus macaques, despite receiving suppressive (n = 7) or interrupted (n = 8) ART, brain tissues had similar SIV-encoded RNA and total and integrated DNA levels compared to brains from infected animals without ART (n = 3). SIV and HIV-1 capsid antigens were immunodetected in brain, principally in microglia/macrophages, regardless of ART duration and outcome. Antiviral immune responses were comparable in the brains of ART-treated and untreated HIV- and SIV-infected hosts. Both HIV-1 and SIV persist in brain tissues despite contemporary ART, with undetectable virus in blood. ART interruption exerted minimal effect on the SIV brain reservoir and did not alter the neuroimmune response profile. These studies underscore the importance of augmenting ART potency in different tissue compartments. IMPORTANCE Antiretroviral therapy (ART) suppresses HIV-1 in plasma and CSF to undetectable levels. However, the impact of contemporary ART on HIV-1 brain reservoirs remains uncertain. An active viral reservoir in the brain during ART could lead to rebound systemic infection after cessation of therapy, development of drug resistance mutations, and neurological disease. ART's impact, including its interruption, on brain proviral DNA remains unclear. The present studies show that in different experimental platforms, contemporary ART did not suppress viral burden in the brain, regardless of ART component regimen, the duration of therapy, and its interruption. Thus, new strategies for effective HIV-1 suppression in the brain are imperative to achieve sustained HIV suppression.
Keywords: ART; central nervous system infections; human immunodeficiency virus; simian immunodeficiency virus; viral reservoirs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Dow SW, Poss ML, Hoover EA. 1990. Feline immunodeficiency virus: a neurotropic lentivirus. J Acquir Immune Defic Syndr (1988) 3:658–668. - PubMed
-
- McClure JJ, Lindsay WA, Taylor W, Ochoa R, Issel CJ, Coulter SJ. 1982. Ataxia in four horses with equine infectious anemia. J Am Vet Med Assoc 180:279–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

