Combining Ivacaftor and Intensive Antibiotics Achieves Limited Clearance of Cystic Fibrosis Infections
- PMID: 34903059
- PMCID: PMC8669489
- DOI: 10.1128/mbio.03148-21
Combining Ivacaftor and Intensive Antibiotics Achieves Limited Clearance of Cystic Fibrosis Infections
Abstract
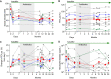
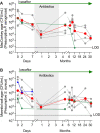
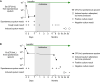
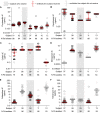
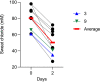
Drugs called CFTR modulators improve the physiologic defect underlying cystic fibrosis (CF) and alleviate many disease manifestations. However, studies to date indicate that chronic lung infections that are responsible for most disease-related mortality generally persist. Here, we investigated whether combining the CFTR modulator ivacaftor with an intensive 3.5-month antibiotic course could clear chronic Pseudomonas aeruginosa or Staphylococcus aureus lung infections in subjects with R117H-CFTR, who are highly ivacaftor-responsive. Ivacaftor alone improved CFTR activity, and lung function and inflammation within 48 h, and reduced P. aeruginosa and S. aureus pathogen density by ∼10-fold within a week. Antibiotics produced an additional ∼10-fold reduction in pathogen density, but this reduction was transient in subjects who remained infected. Only 1/5 P. aeruginosa-infected and 1/7 S. aureus-infected subjects became persistently culture-negative after the combined treatment. Subjects appearing to clear infection did not have particularly favorable baseline lung function or inflammation, pathogen density or antibiotic susceptibility, or bronchiectasis scores on CT scans, but they did have remarkably low sweat chloride values before and after ivacaftor. All persistently P. aeruginosa-positive subjects remained infected by their pretreatment strain, whereas subjects persistently S. aureus-positive frequently lost and gained strains. This work suggests chronic CF infections may resist eradication despite marked and rapid modulator-induced improvements in lung infection and inflammation parameters and aggressive antibiotic treatment. IMPORTANCE Recent work shows that people with CF and chronic lung infections generally remain persistently infected after treatment with drugs that target the CF physiological defect (called CFTR modulators). However, changes produced by modulators could increase antibiotic efficacy. We tested the approach of combining modulators and intensive antibiotics in rapid succession and found that while few subjects cleared their infections, combined treatment appeared most effective in subjects with the highest CFTR activity. These findings highlight challenges that remain to improve the health of people with CF.
Keywords: CFTR modulator; Pseudomonas aeruginosa; Staphylococcus aureus; cystic fibrosis; lung infection.
Conflict of interest statement
The authors declare a conflict of interest. Some specimen collection was funded by an unrestricted investigator-initiated research grant from Vertex, Inc. No sponsor (including Vertex) influenced the study design, data analysis or interpretation, decision to publish, manuscript content, or approved the manuscript. E.F.M. reports grants and personal fees from Vertex, Inc., during the conduct of the study as well participation in Vertex clinical trials for which his institute receives payment. P.K.S. reports an unrestricted investigator-initiated research grant from Vertex, Inc., unrelated to this study.
Figures



References
-
- Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordoñez C, Elborn JS, VX08-770-102 Study Group. 2011. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 365:1663–1672. doi:10.1056/NEJMoa1105185. - DOI - PMC - PubMed
-
- Hisert KB, Heltshe SL, Pope C, Jorth P, Wu X, Edwards RM, Radey M, Accurso FJ, Wolter DJ, Cooke G, Adam RJ, Carter S, Grogan B, Launspach JL, Donnelly SC, Gallagher CG, Bruce JE, Stoltz DA, Welsh MJ, Hoffman LR, McKone EF, Singh PK. 2017. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med 195:1617–1628. doi:10.1164/rccm.201609-1954OC. - DOI - PMC - PubMed
-
- Durfey SL, McGeer K, Ratjen AM, Carter SC, Grogan B, Gallagher CG, Stoltz DA, Hoffman L, Welsh MJ, McKone E. 2019. Six-year follow-up of ivacaftor-treated subjects with CFTR-G551D: an update on the Dublin cohort. Pediatr Pulmonol 54:S334–S334.
-
- Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, Sagel SD, Khan U, Mayer-Hamblett N, Van Dalfsen JM, Joseloff E, Ramsey BW. 2014. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 190:175–184. doi:10.1164/rccm.201404-0703OC. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

