Functional recovery following hospitalisation of patients diagnosed with COVID-19: a protocol for a longitudinal cohort study
- PMID: 34903545
- PMCID: PMC8671848
- DOI: 10.1136/bmjopen-2021-053021
Functional recovery following hospitalisation of patients diagnosed with COVID-19: a protocol for a longitudinal cohort study
Abstract
Introduction: COVID-19 is an international public health crisis with more than 132 million infections worldwide. Beyond acute infection, emerging data indicate patients diagnosed with COVID-19 may experience persistent sequelae similar to survivors of sepsis or acute respiratory syndromes, including mobility limitations and fatigue. However, there is limited evidence on the trajectory of functional recovery in those hospitalised with COVID-19. The primary aim of the Coronavirus Registry Functional Recovery (COREG-FR) study is to understand the trajectory of functional recovery among individuals hospitalised for COVID-19 over the medium (up to 6 months) and longer term (6-12 months) that will guide clinical care and optimal management of serious COVID-19 illness and recovery.
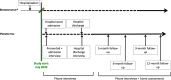
Methods and analysis: COREG-FR is a multicentre longitudinal cohort study. We will enrol a minimum of 211 adults age 18 years and older with COVID-19 from five hospitals. Participants will be followed from admission to hospital as an inpatient, to hospital discharge, and at 3-month, 6-month, 9-month and up to 12-month post-hospital discharge. We will conduct telephone interviews at ward admission and discharge, and telephone interviews plus in-person assessments of physical function and lung function at all remaining follow-ups. Our primary outcome is the Activity Measure for Post-Acute Care mobility scale measured at all time points. We will conduct linear mixed effects regression analyses to explore determinants of functional outcomes after COVID-19 illness. Subgroup analyses based on age (≤65 vs >65 years), frailty status (Clinical Frailty Scale score ≤4 vs >5) and variants of concern will be conducted.
Ethics and dissemination: COREG-FR has been approved by Research Ethics Boards at participating sites. We will disseminate this work through peer-reviewed manuscripts, presentations at national and international meetings and through the established COREG website (www.coregontario.ca). COREG-FR is designed as a data platform for future studies evaluating COVID-19 recovery.
Trial registration number: NCT04602260; Pre-results.
Keywords: COVID-19; epidemiology; protocols & guidelines; rehabilitation medicine.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Organization WH . WHO Coronavirus Disease (COVID-19) Dashboard. Secondary WHO Coronavirus Disease (COVID-19) Dashboard, 2021. Available: https://covid19.who.int
-
- Ontario Go . COVID-19 (coronavirus) in Ontario. secondary COVID-19 (coronavirus) in Ontario. Available: https://covid-19.ontario.ca/data
-
- Ontario PH . Ontario COVID-19 data tool. secondary Ontario COVID-19 data tool, 2021. Available: https://www.publichealthontario.ca/en/data-and-analysis/infectious-disea...
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
