An ionizable lipid toolbox for RNA delivery
- PMID: 34903741
- PMCID: PMC8668901
- DOI: 10.1038/s41467-021-27493-0
An ionizable lipid toolbox for RNA delivery
Abstract
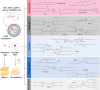
Recent years have witnessed incredible growth in RNA therapeutics, which has benefited significantly from decades of research on lipid nanoparticles, specifically its key component—the ionizable lipid. This comment discusses the major ionizable lipid types, and provides perspectives for future development.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Han X, Mitchell MJ, Nie G. Nanomaterials for therapeutic RNA delivery. Matter. 2020;3:1948–1975.
-
- Swingle KL, Hamilton AG, Mitchell MJ. Lipid nanoparticle-mediated delivery of mRNA therapeutics and vaccines. Trends Mol. Med. 2021;27:616–617. - PubMed
-
- Semple SC, et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010;28:172–176. - PubMed
-
- Heyes J, Palmer L, Bremner K, MacLachlan I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J. Control. Release. 2005;107:276–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

