Generation of mutant pigs by lipofection-mediated genome editing in embryos
- PMID: 34903813
- PMCID: PMC8668999
- DOI: 10.1038/s41598-021-03325-5
Generation of mutant pigs by lipofection-mediated genome editing in embryos
Abstract
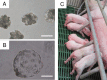
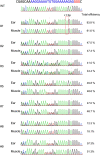
The specificity and efficiency of CRISPR/Cas9 gene-editing systems are determined by several factors, including the mode of delivery, when applied to mammalian embryos. Given the limited time window for delivery, faster and more reliable methods to introduce Cas9-gRNA ribonucleoprotein complexes (RNPs) into target embryos are needed. In pigs, somatic cell nuclear transfer using gene-modified somatic cells and the direct introduction of gene editors into the cytoplasm of zygotes/embryos by microinjection or electroporation have been used to generate gene-edited embryos; however, these strategies require expensive equipment and sophisticated techniques. In this study, we developed a novel lipofection-mediated RNP transfection technique that does not require specialized equipment for the generation of gene-edited pigs and produced no detectable off-target events. In particular, we determined the concentration of lipofection reagent for efficient RNP delivery into embryos and successfully generated MSTN gene-edited pigs (with mutations in 7 of 9 piglets) after blastocyst transfer to a recipient gilt. This newly established lipofection-based technique is still in its early stages and requires improvements, particularly in terms of editing efficiency. Nonetheless, this practical method for rapid and large-scale lipofection-mediated gene editing in pigs has important agricultural and biomedical applications.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

