Different Respiratory Samples for COVID-19 Detection by Standard and Direct Quantitative RT-PCR: A Literature Review
- PMID: 34903989
- PMCID: PMC8653661
- DOI: 10.22037/ijpr.2021.115458.15383
Different Respiratory Samples for COVID-19 Detection by Standard and Direct Quantitative RT-PCR: A Literature Review
Abstract
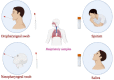
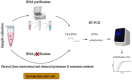
The most common diagnostic method for detecting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is real-time quantitative reverse transcriptase-polymerase chain reaction (RT-qPCR). Upper respiratory tract samples, including nasopharyngeal swab (NPS), oropharyngeal swab (OPS), saliva and lower respiratory tract samples such as sputum, are the most widely used specimens for diagnosis of SARS-CoV-2 using RT-qPCR. This study aimed to compare the diagnostic performance of different samples for Coronavirus disease 2019 (COVID-19) detection. It was found that NPS, the reference respiratory specimen for COVID-19 detection, is more sensitive than OPS. However, the application of NPS has many drawbacks, including challenging sampling process and increased risk of transmission to healthcare workers (HCWs). Saliva samples can be collected less invasively and quickly by HCWs with less contact or by own patients, and they can be considered as an alternative to NPS for COVID-19 detection by RT-qPCR. Additionally, sputum, which demonstrates higher viral load can be applied in patients with productive coughs and negative results from NPS. Commonly, after viral RNA purification from patient samples, which is time-consuming and costly, RT-qPCR is performed to diagnose SARS-CoV-2. Herein, different approaches including physical (heat inactivation) and chemical (proteinase K treatment) methods, used in RNA extraction free- direct RT-qPCR, were reviewed. The results of direct RT-qPCR assays were comparable to the results of standard RT-qPCR, while cost and time were saved. However, optimal protocol to decrease cost and processing time, proper transport medium and detection kit should be determined.
Keywords: COVID-19; Nasopharyngeal swab; Oropharyngeal swab; RT-qPCR; SARS-CoV-2; Sputum; saliva.
Figures
Similar articles
-
Clinical Evaluation of Nasopharyngeal, Oropharyngeal, Nasal Swabs, and Saliva for the Detection of SARS-CoV-2 by Direct RT-PCR.Diagnostics (Basel). 2022 Apr 27;12(5):1091. doi: 10.3390/diagnostics12051091. Diagnostics (Basel). 2022. PMID: 35626247 Free PMC article.
-
Saliva as a testing specimen with or without pooling for SARS-CoV-2 detection by multiplex RT-PCR test.PLoS One. 2021 Feb 23;16(2):e0243183. doi: 10.1371/journal.pone.0243183. eCollection 2021. PLoS One. 2021. PMID: 33621263 Free PMC article.
-
Comparable detection of nasopharyngeal swabs and induced sputum specimens for viral nucleic acid detection of suspected novel coronavirus (SARS-Cov-2) patients in Fayoum governorate, Egypt.Beni Suef Univ J Basic Appl Sci. 2023;12(1):43. doi: 10.1186/s43088-023-00379-4. Epub 2023 May 2. Beni Suef Univ J Basic Appl Sci. 2023. PMID: 37151720 Free PMC article.
-
Diagnosis of SARS-Cov-2 Infection by RT-PCR Using Specimens Other Than Naso- and Oropharyngeal Swabs: A Systematic Review and Meta-Analysis.Diagnostics (Basel). 2021 Feb 21;11(2):363. doi: 10.3390/diagnostics11020363. Diagnostics (Basel). 2021. PMID: 33670020 Free PMC article. Review.
-
Saliva as an alternative specimen to nasopharyngeal swabs for COVID-19 diagnosis: Review.Access Microbiol. 2022 May 20;4(5):acmi000366. doi: 10.1099/acmi.0.000366. eCollection 2022 Aug. Access Microbiol. 2022. PMID: 36003360 Free PMC article. Review.
Cited by
-
Comprehensive Review of COVID-19: Epidemiology, Pathogenesis, Advancement in Diagnostic and Detection Techniques, and Post-Pandemic Treatment Strategies.Int J Mol Sci. 2024 Jul 26;25(15):8155. doi: 10.3390/ijms25158155. Int J Mol Sci. 2024. PMID: 39125722 Free PMC article. Review.
-
Conventional and Novel Diagnostic Tools for the Diagnosis of Emerging SARS-CoV-2 Variants.Vaccines (Basel). 2023 Feb 6;11(2):374. doi: 10.3390/vaccines11020374. Vaccines (Basel). 2023. PMID: 36851252 Free PMC article. Review.
-
SARS-CoV-2 Burden in Wastewater and its Elimination Using Disinfection.Microbiol Insights. 2023 Sep 22;16:11786361231201598. doi: 10.1177/11786361231201598. eCollection 2023. Microbiol Insights. 2023. PMID: 37745090 Free PMC article. Review.
-
Do COVID-19 CT features vary between patients from within and outside mainland China? Findings from a meta-analysis.Front Public Health. 2022 Oct 14;10:939095. doi: 10.3389/fpubh.2022.939095. eCollection 2022. Front Public Health. 2022. PMID: 36311632 Free PMC article.
-
Flexible upscaling of laboratory PCR testing capacity at the Robert Koch Institute during the SARS-CoV-2 pandemic.Virol J. 2023 Jul 5;20(1):139. doi: 10.1186/s12985-023-02088-x. Virol J. 2023. PMID: 37408040 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
