Paricalcitol in hemodialysis patients with secondary hyperparathyroidism and its potential benefits
- PMID: 34904087
- PMCID: PMC8638032
- DOI: 10.12998/wjcc.v9.i33.10172
Paricalcitol in hemodialysis patients with secondary hyperparathyroidism and its potential benefits
Abstract
Background: Secondary hyperparathyroidism (SHPT) is a common complication in patients with end-stage renal disease and it is also common in hemodialysis patients. SHPT can increase bone fragility and calcification of blood vessels and soft tissues, which greatly increases the risk of death.
Aim: To discuss the outcome, safety and other potential benefits of paricalcitol injection in hemodialysis patients with SHPT.
Methods: We recruited 40 patients who received hemodialysis at our hospital for chronic renal failure with SHPT between March and December 2019. They received paricalcitol injection for 24 wk (starting dose, 0.06-0.08 μg/kg), three times per week. They were followed up at the baseline (week 0), week 4, week 12 and week 24. The primary outcome indicator was the percentage of patients with a > 30% decrease in intact parathyroid hormone (iPTH) levels at week 24 compared with the baseline. The secondary outcome indicators included percentage decrease in iPTH levels at week 24, standard-reaching rate of iPTH (percentage of patients with iPTH down to 130-585 pg/mL), changes in serum levels of calcium (Ca), phosphate (P), Ca × P product, alkaline phosphatase (ALP), creatinine (Cre), hemoglobin (Hb), and C-reactive protein (CRP), and incidence of adverse events (AEs).
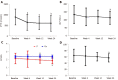
Results: After 24 wk of treatment, iPTH levels decreased significantly (598.88 ± 381.29 pg/mL vs 888.84 ± 376.88 pg/mL, P < 0.05). More than 30% decrease of iPTH was found in 21 of 36 (58.33%) patients. The average decrease in iPTH levels was 32.16 ± 4.33%; the standard-reaching rate of iPTH levels was 66.67% (24/36); and ALP levels decreased significantly compared with the baseline (113.72 ± 41.73 IU/L vs 133.45 ± 56.86 IU/L) (t = 2.798, P < 0.05). There were no significant differences in the serum levels of calcium, Hb, Cre and CRP compared with the baseline (P > 0.05). After 24 wk of treatment, serum P levels decreased compared with the baseline (1.91 ± 0.40 mmol/L vs 2.16 ± 0.66 mmol/L) (t = 2.830, P < 0.05). Ca × P product decreased significantly compared with the baseline (56.38 ± 13.22 mg2/dL2 vs 63.97 ± 20.30 mg2/dL2) (t = 2.717, P < 0.05). No serious adverse events occurred.
Conclusion: Paricalcitol was a safe and effective treatment for hemodialysis patients with SHPT. It decreased serum levels of iPTH, ALP and P and maintained stability of serum Ca levels.
Keywords: Drug efficacy; Drug safety; Hemodialysis; Paricalcitol; Secondary hyperparathyroidism.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declared that they have no conflicts of interest to this work.
Figures
References
-
- Messa P, Alfieri CM. Secondary and Tertiary Hyperparathyroidism. Front Horm Res. 2019;51:91–108. - PubMed
-
- Goodman WG, Quarles LD. Development and progression of secondary hyperparathyroidism in chronic kidney disease: lessons from molecular genetics. Kidney Int. 2008;74:276–288. - PubMed
-
- Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. - PubMed
-
- Mizobuchi M, Ogata H, Koiwa F. Secondary Hyperparathyroidism: Pathogenesis and Latest Treatment. Ther Apher Dial. 2019;23:309–318. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

