Deletion of SA β-Gal+ cells using senolytics improves muscle regeneration in old mice
- PMID: 34904366
- PMCID: PMC8761017
- DOI: 10.1111/acel.13528
Deletion of SA β-Gal+ cells using senolytics improves muscle regeneration in old mice
Abstract
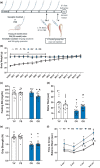
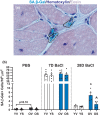
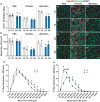
Systemic deletion of senescent cells leads to robust improvements in cognitive, cardiovascular, and whole-body metabolism, but their role in tissue reparative processes is incompletely understood. We hypothesized that senolytic drugs would enhance regeneration in aged skeletal muscle. Young (3 months) and old (20 months) male C57Bl/6J mice were administered the senolytics dasatinib (5 mg/kg) and quercetin (50 mg/kg) or vehicle bi-weekly for 4 months. Tibialis anterior (TA) was then injected with 1.2% BaCl2 or PBS 7- or 28 days prior to euthanization. Senescence-associated β-Galactosidase positive (SA β-Gal+) cell abundance was low in muscle from both young and old mice and increased similarly 7 days following injury in both age groups, with no effect of D+Q. Most SA β-Gal+ cells were also CD11b+ in young and old mice 7- and 14 days following injury, suggesting they are infiltrating immune cells. By 14 days, SA β-Gal+/CD11b+ cells from old mice expressed senescence genes, whereas those from young mice expressed higher levels of genes characteristic of anti-inflammatory macrophages. SA β-Gal+ cells remained elevated in old compared to young mice 28 days following injury, which were reduced by D+Q only in the old mice. In D+Q-treated old mice, muscle regenerated following injury to a greater extent compared to vehicle-treated old mice, having larger fiber cross-sectional area after 28 days. Conversely, D+Q blunted regeneration in young mice. In vitro experiments suggested D+Q directly improve myogenic progenitor cell proliferation. Enhanced physical function and improved muscle regeneration demonstrate that senolytics have beneficial effects only in old mice.
Keywords: regeneration; satellite cells; senescence; senolytics; skeletal muscle.
© 2021 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
Z.J.T., Z.L., A.F., and M.F. are employees of Boehringer Ingelheim Pharmaceuticals, Inc. All other authors have no financial interests.
Figures



References
-
- Baker, D. J. , Childs, B. G. , Durik, M. , Wijers, M. E. , Sieben, C. J. , Zhong, J. , A. Saltness, R. , Jeganathan, K. B. , Verzosa, G. C. , Pezeshki, A. , Khazaie, K. , Miller, J. D. , & van Deursen, J. M. (2016). Naturally occurring p16(Ink4a)‐positive cells shorten healthy lifespan. Nature, 530(7589), 184–189. 10.1038/nature16932 - DOI - PMC - PubMed
-
- Basisty, N. , Kale, A. , Jeon, O. H. , Kuehnemann, C. , Payne, T. , Rao, C. , Holtz, A. , Shah, S. , Sharma, V. , Ferrucci, L. , Campisi, J. , & Schilling, B. (2020). A proteomic atlas of senescence‐associated secretomes for aging biomarker development. PLoS Biology, 18(1), e3000599. 10.1371/journal.pbio.3000599 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

