Short-Term Parafoveal Cone Loss Despite Preserved Ellipsoid Zone in Rod Cone Dystrophy
- PMID: 34904999
- PMCID: PMC8684316
- DOI: 10.1167/tvst.10.14.11
Short-Term Parafoveal Cone Loss Despite Preserved Ellipsoid Zone in Rod Cone Dystrophy
Abstract
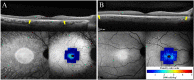
Purpose: Rod-cone dystrophy (RCD) is characterized by centripetal loss of rod followed by cone photoreceptors. In this prospective, observational cohort, we used flood-illumination adaptive optics (AO) imaging to investigate parafoveal cone loss in regions with preserved ellipsoid zone (EZ) in patients with RCD.
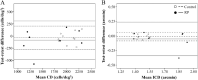
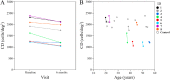
Methods: Eight patients with RCD and 10 age-matched healthy controls underwent spectral-domain optical coherence tomography and AO imaging. The RCD cohort underwent a follow-up examination after 6 months. Cone density (CD) and intercone distance (ICD) measurements were performed at 2° temporal from the fovea. Baseline CD and ICD values were compared between the control and patient groups, and longitudinal changes were calculated in the patient group. Residual EZ span in patients was measured in horizontal foveal B-scans.
Results: Between the control and patient groups, there was no significant difference in the baseline CD (2094 vs. 1750 cells/deg2, respectively; P = 0.09) and ICD (1.46 vs. 1.62 arcmin, respectively; P = 0.08). Mean CD declined by 198 cells/deg2 (-11.3%; P < 0.01), and mean ICD increased by 0.09 arcmin (+5.6%; P = 0.01) at the 6-month follow-up in the patient group. Mean baseline and follow-up residual EZ spans in the six patients with EZ defect were 3189 µm and 3065 µm, respectively (-3.9%; P = 0.08).
Conclusions: AO imaging detected significant parafoveal cone loss over 6-month follow-up even in regions with preserved EZ. Further studies to refine AO imaging protocol and validate cone metrics as a structural endpoint in early RCD are warranted.
Translational relevance: CD and ICD may change prior to EZ span shortening in RCD.
Conflict of interest statement
Disclosure:
Figures



References
-
- Roshandel D, Thompson JA, Charng J, et al. .. Exploring microperimetry and autofluorescence endpoints for monitoring disease progression in PRPF31-associated retinopathy. Ophthalmic Genet . 2020; 42: 1–14. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

