Anticancer Drugs Approved by the US Food and Drug Administration From 2009 to 2020 According to Their Mechanism of Action
- PMID: 34905002
- PMCID: PMC8672232
- DOI: 10.1001/jamanetworkopen.2021.38793
Anticancer Drugs Approved by the US Food and Drug Administration From 2009 to 2020 According to Their Mechanism of Action
Abstract
Importance: Both novel and next-in-class cancer drugs have a role in oncology, but the relative development of each is understudied.
Objective: To characterize the mechanisms of action of anticancer drugs approved by the US Food and Drug Administration (FDA) between 2009 and 2020, noting how many approvals were based on a new mechanism of action vs next-in-class approvals.
Design, study, and participants: This cross-sectional study included all anticancer drugs approved by the FDA from January 2009 to December 2020. The mechanism of action of each drug was extracted from FDA labels. Supportive-care treatments were excluded.
Exposures: Name of drug approved, date of approval, indication, tumor type, mechanism of action, broad pharmaceutical class, and biological target. Approvals considering all tumor types and each tumor type separately were classified in 3 nonoverlapping categories: new mechanism of action, next in class, or subsequent approval.
Main outcomes and measures: The number of all approvals each year; the number of approvals based on a new mechanism of action, either by drug (considering all tumor types) or by indication (considering tumor types separately); and the frequency of these numbers over time.
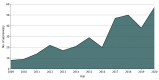
Results: Overall, 332 approvals were included. Between 2009 and 2020, there was an increase in the total number of approvals from 8 to 57. We found that 209 approvals (63%) were for a next-in-class indication in a new tumor type (84 [25%]) or a subsequent indication of the same drug in the same tumor type (195 [59%]). When considering each tumor type separately, 123 approvals (37%) were based on a new mechanism of action.
Conclusions and relevance: In this study, approvals based on a new mechanism of action represented a minority of all approvals. Further consideration of incentives for drug development are needed to prioritize novel or highly innovative and transformative anticancer drugs.
Conflict of interest statement
Figures


Comment in
-
Anticancer Drug Approvals in the Past Decade-Quality vs Quantity.JAMA Netw Open. 2021 Dec 1;4(12):e2139178. doi: 10.1001/jamanetworkopen.2021.39178. JAMA Netw Open. 2021. PMID: 34905012 No abstract available.

