Targeting radiation-tolerant persister cells as a strategy for inhibiting radioresistance and recurrence in glioblastoma
- PMID: 34905060
- PMCID: PMC9248405
- DOI: 10.1093/neuonc/noab288
Targeting radiation-tolerant persister cells as a strategy for inhibiting radioresistance and recurrence in glioblastoma
Abstract
Background: Compelling evidence suggests that glioblastoma (GBM) recurrence results from the expansion of a subset of tumor cells with robust intrinsic or therapy-induced radioresistance. However, the mechanisms underlying GBM radioresistance and recurrence remain elusive. To overcome obstacles in radioresistance research, we present a novel preclinical model ideally suited for radiobiological studies.
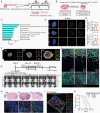
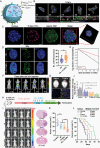
Methods: With this model, we performed a screen and identified a radiation-tolerant persister (RTP) subpopulation. RNA sequencing was performed on RTP and parental cells to obtain mRNA and miRNA expression profiles. The regulatory mechanisms among NF-κB, YY1, miR-103a, XRCC3, and FGF2 were investigated by transcription factor activation profiling array analysis, chromatin immunoprecipitation, western blot analysis, luciferase reporter assays, and the MirTrap system. Transferrin-functionalized nanoparticles (Tf-NPs) were employed to improve blood-brain barrier permeability and RTP targeting.
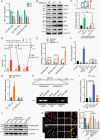
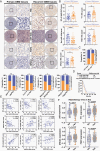
Results: RTP cells drive radioresistance by preferentially activating DNA damage repair and promoting stemness. Mechanistic investigations showed that continual radiation activates the NF-κB signaling cascade and promotes nuclear translocation of p65, leading to enhanced expression of YY1, the transcription factor that directly suppresses miR-103a transcription. Restoring miR-103a expression under these conditions suppressed the FGF2-XRCC3 axis and decreased the radioresistance capability. Moreover, Tf-NPs improved radiosensitivity and provided a significant survival benefit.
Conclusions: We suggest that the NF-κB-YY1-miR-103a regulatory axis is indispensable for the function of RTP cells in driving radioresistance and recurrence. Thus, our results identified a novel strategy for improving survival in patients with recurrent/refractory GBM.
Keywords: DNA damage repair; glioblastoma; glioma stem cell; radioresistance.
Published by Oxford University Press on behalf of the Society for Neuro-Oncology 2021.
Figures



Comment in
-
Targeting adaptive radioresistance in glioblastoma.Neuro Oncol. 2022 Jul 1;24(7):1071-1073. doi: 10.1093/neuonc/noac074. Neuro Oncol. 2022. PMID: 35323979 Free PMC article. No abstract available.
References
-
- Tirosh I, Suvà ML. Tackling the many facets of glioblastoma heterogeneity. Cell Stem Cell. 2020;26(3):303–304. - PubMed
-
- Sulman EP, Ismaila N, Armstrong TS, et al. . Radiation therapy for glioblastoma: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Guideline. J Clin Oncol. 2017;35(3):361–369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

