Antigenic characterization of influenza and SARS-CoV-2 viruses
- PMID: 34905077
- PMCID: PMC8669429
- DOI: 10.1007/s00216-021-03806-6
Antigenic characterization of influenza and SARS-CoV-2 viruses
Abstract
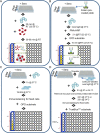
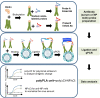
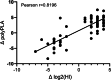
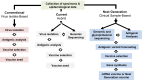
Antigenic characterization of emerging and re-emerging viruses is necessary for the prevention of and response to outbreaks, evaluation of infection mechanisms, understanding of virus evolution, and selection of strains for vaccine development. Primary analytic methods, including enzyme-linked immunosorbent/lectin assays, hemagglutination inhibition, neuraminidase inhibition, micro-neutralization assays, and antigenic cartography, have been widely used in the field of influenza research. These techniques have been improved upon over time for increased analytical capacity, and some have been mobilized for the rapid characterization of the SARS-CoV-2 virus as well as its variants, facilitating the development of highly effective vaccines within 1 year of the initially reported outbreak. While great strides have been made for evaluating the antigenic properties of these viruses, multiple challenges prevent efficient vaccine strain selection and accurate assessment. For influenza, these barriers include the requirement for a large virus quantity to perform the assays, more than what can typically be provided by the clinical samples alone, cell- or egg-adapted mutations that can cause antigenic mismatch between the vaccine strain and circulating viruses, and up to a 6-month duration of vaccine development after vaccine strain selection, which allows viruses to continue evolving with potential for antigenic drift and, thus, antigenic mismatch between the vaccine strain and the emerging epidemic strain. SARS-CoV-2 characterization has faced similar challenges with the additional barrier of the need for facilities with high biosafety levels due to its infectious nature. In this study, we review the primary analytic methods used for antigenic characterization of influenza and SARS-CoV-2 and discuss the barriers of these methods and current developments for addressing these challenges.
Keywords: Antigenic analysis; Antigenic characterization; Antigenic drift; Influenza; SARS-CoV-2; Vaccine strain selection.
© 2021. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Prevention CfDCa. Disease burden of influenza October 5, 2020 [Available from: https://www.cdc.gov/flu/about/burden/index.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

