Clinical Pharmacokinetics and Pharmacodynamics of Roxadustat
- PMID: 34905154
- PMCID: PMC8891203
- DOI: 10.1007/s40262-021-01095-x
Clinical Pharmacokinetics and Pharmacodynamics of Roxadustat
Abstract
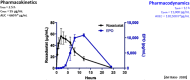
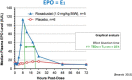
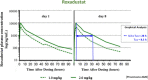
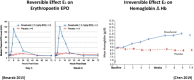
The pharmacokinetics of roxadustat are well characterized, with an apparent volume of distribution after oral administration of 22-57 L, apparent clearance of 1.2-2.65 L/h, and renal clearance of 0.030-0.026 L/h in healthy volunteers; the elimination half-life is 9.6-16 h. Plasma binding is 99% and the fraction eliminated by hemodialysis is 2.34%. As an interpretation of the pharmacodynamics of roxadustat, we proposed a concept with a hypothetical cascade of two subsequent effects, first on erythropoetin (EPO) and second on hemoglobin (delta Hb). The primary effect on EPO is observed within a few hours after roxadustat administration and can be modeled using the sigmoidal Hill equation. The concentration at half-maximum effect can be inferred at 10-36 µg/mL, the Hill coefficient at 3.3, and the effect bisection time at 10-17 h, corresponding to EPO half-life. The subsequent effect on hemoglobin (delta Hb) is observed after several weeks and can be interpreted as an irreversible, dose proportional, unsaturable effect, continuing in agreement with the lifespan of red blood cells of 63-112 days.
© 2021. The Author(s).
Conflict of interest statement
David Czock and Frieder Keller have no financial or intellectual conflicts of interest to declare.
Figures
Similar articles
-
Effect of Moderate Hepatic Impairment on the Pharmacokinetics and Pharmacodynamics of Roxadustat, an Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor.Clin Drug Investig. 2016 Sep;36(9):743-751. doi: 10.1007/s40261-016-0422-y. Clin Drug Investig. 2016. PMID: 27352308 Free PMC article.
-
Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat (FG-4592) for Treatment of Anemia in Chronic Kidney Disease: A Placebo-Controlled Study of Pharmacokinetic and Pharmacodynamic Profiles in Hemodialysis Patients.J Clin Pharmacol. 2020 Nov;60(11):1432-1440. doi: 10.1002/jcph.1648. Epub 2020 Jun 30. J Clin Pharmacol. 2020. PMID: 32603526 Free PMC article. Clinical Trial.
-
Effect of Kidney Function and Dialysis on the Pharmacokinetics and Pharmacodynamics of Roxadustat, an Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor.Eur J Drug Metab Pharmacokinet. 2021 Jan;46(1):141-153. doi: 10.1007/s13318-020-00658-w. Eur J Drug Metab Pharmacokinet. 2021. PMID: 33165773 Free PMC article. Clinical Trial.
-
The efficacy and safety of roxadustat treatment for anemia in patients with kidney disease: a meta-analysis and systematic review.Int Urol Nephrol. 2021 May;53(5):985-997. doi: 10.1007/s11255-020-02693-7. Epub 2021 Jan 3. Int Urol Nephrol. 2021. PMID: 33389461
-
Roxadustat for the treatment of anemia in patients with chronic kidney diseases: a meta-analysis.Aging (Albany NY). 2021 Jun 11;13(13):17914-17929. doi: 10.18632/aging.203143. Epub 2021 Jun 11. Aging (Albany NY). 2021. PMID: 34115611 Free PMC article.
Cited by
-
Bioanalysis, Analysis, Chemistry, and Pharmacological Aspects of Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors.Curr Top Med Chem. 2025;25(12):1451-1466. doi: 10.2174/0115680266324419241227102847. Curr Top Med Chem. 2025. PMID: 39791153 Review.
-
Case report: Roxadustat overdose in an anemia patient of chronic kidney disease: insight beyond insignificant consequence.Front Nephrol. 2024 Aug 2;4:1413496. doi: 10.3389/fneph.2024.1413496. eCollection 2024. Front Nephrol. 2024. PMID: 39155928 Free PMC article.
-
A critical review of Roxadustat formulations, solid state studies, and analytical methodology.Heliyon. 2023 Jun 1;9(6):e16595. doi: 10.1016/j.heliyon.2023.e16595. eCollection 2023 Jun. Heliyon. 2023. PMID: 37346363 Free PMC article. Review.
-
Roxadustat: Not just for anemia.Front Pharmacol. 2022 Aug 29;13:971795. doi: 10.3389/fphar.2022.971795. eCollection 2022. Front Pharmacol. 2022. PMID: 36105189 Free PMC article. Review.
-
A DNA nanostructure-Hif-1α inducer complex as novel nanotherapy against cisplatin-induced acute kidney injury.Cell Prolif. 2024 Jun;57(6):e13601. doi: 10.1111/cpr.13601. Epub 2024 Jan 14. Cell Prolif. 2024. PMID: 38221742 Free PMC article.
References
-
- Akizawa T, Iwasaki M, Otsuka T, Reusch M, Misumi T. Roxadustat Treatment of Chronic Kidney Disease-Associated Anemia in Japanese Patients Not on Dialysis: A Phase 2, Randomized, Double-Blind Placebo-Controlled Trial. Adv Ther. 2019;36(6):1438–1454. doi: 10.1007/s12325-019-00943-4. - DOI - PMC - PubMed
-
- Akizawa T, Yamaguchi Y, Otsuka T, Reusch M. A Phase 3, Multicenter, Randomized, Two-Arm, Open-Label Study of Intermittent Oral Dosing of Roxadustat for the Treatment of Anemia in Japanese Erythropoiesis-Stimulating Agent-Naïve Chronic Kidney Disease Patients Not on Dialysis. Nephron. 2020;144(8):372–382. doi: 10.1159/000508100. - DOI - PMC - PubMed
-
- Akizawa T, Nangaku M, Yonekawa T, Okuda N, Kawamatsu S, Onoue T, et al. Efficacy and Safety of Daprodustat Compared with Darbepoetin Alfa in Japanese Hemodialysis Patients with Anemia: a randomized, double-blind, Phase 3 Trial. Clin J Am Soc Nephrol. 2020;15(8):1155–1165. doi: 10.2215/CJN.16011219. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

