Nitrogen Atom Transfer Catalysis by Metallonitrene C-H Insertion: Photocatalytic Amidation of Aldehydes
- PMID: 34905281
- PMCID: PMC9305406
- DOI: 10.1002/anie.202115626
Nitrogen Atom Transfer Catalysis by Metallonitrene C-H Insertion: Photocatalytic Amidation of Aldehydes
Abstract
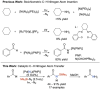
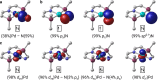
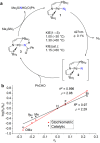
C-H amination and amidation by catalytic nitrene transfer are well-established and typically proceed via electrophilic attack of nitrenoid intermediates. In contrast, the insertion of (formal) terminal nitride ligands into C-H bonds is much less developed and catalytic nitrogen atom transfer remains unknown. We here report the synthesis of a formal terminal nitride complex of palladium. Photocrystallographic, magnetic, and computational characterization support the assignment as an authentic metallonitrene (Pd-N) with a diradical nitrogen ligand that is singly bonded to PdII . Despite the subvalent nitrene character, selective C-H insertion with aldehydes follows nucleophilic selectivity. Transamidation of the benzamide product is enabled by reaction with N3 SiMe3 . Based on these results, a photocatalytic protocol for aldehyde C-H trimethylsilylamidation was developed that exhibits inverted, nucleophilic selectivity as compared to typical nitrene transfer catalysis. This first example of catalytic C-H nitrogen atom transfer offers facile access to primary amides after deprotection.
Keywords: Amides; C−H Activation; Nitrenes; Palladium; Photocatalysis.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

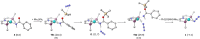

Similar articles
-
A platinum(II) metallonitrene with a triplet ground state.Nat Chem. 2020 Nov;12(11):1054-1059. doi: 10.1038/s41557-020-0522-4. Epub 2020 Aug 24. Nat Chem. 2020. PMID: 32839602
-
Transition Metal-Catalyzed Regioselective Direct C-H Amidation: Interplay between Inner- and Outer-Sphere Pathways for Nitrene Cross-Coupling Reactions.Acc Chem Res. 2022 Aug 2;55(15):2123-2137. doi: 10.1021/acs.accounts.2c00283. Epub 2022 Jul 19. Acc Chem Res. 2022. PMID: 35853135
-
Nitrene Radical Intermediates in Catalytic Synthesis.Chemistry. 2017 Oct 9;23(56):13819-13829. doi: 10.1002/chem.201702537. Epub 2017 Sep 14. Chemistry. 2017. PMID: 28675476 Free PMC article.
-
Skeletal Editing via Transition-Metal-Catalyzed Nitrene Insertion.Chem Rec. 2024 Dec;24(12):e202400184. doi: 10.1002/tcr.202400184. Epub 2024 Nov 28. Chem Rec. 2024. PMID: 39607383 Review.
-
Prospects and challenges for nitrogen-atom transfer catalysis.Nat Rev Chem. 2023 Jun;7(6):424-438. doi: 10.1038/s41570-023-00482-1. Epub 2023 Apr 13. Nat Rev Chem. 2023. PMID: 37117815 Review.
Cited by
-
Cationic Divalent Metal Sites (M = Mn, Fe, Co) Operating as Both Nitrene-Transfer Agents and Lewis Acids toward Mediating the Synthesis of Three- and Five-Membered N-Heterocycles.Inorg Chem. 2023 Jul 10;62(27):10743-10761. doi: 10.1021/acs.inorgchem.3c01209. Epub 2023 Jun 23. Inorg Chem. 2023. PMID: 37352838 Free PMC article.
-
C=C Dissociative Imination of Styrenes by a Photogenerated Metallonitrene.JACS Au. 2024 Sep 3;4(9):3421-3426. doi: 10.1021/jacsau.4c00571. eCollection 2024 Sep 23. JACS Au. 2024. PMID: 39328761 Free PMC article.
-
Photoinitiated Single-Crystal to Single-Crystal Redox Transformations of Titanium-Oxo Clusters.J Am Chem Soc. 2024 Jun 26;146(25):17325-17333. doi: 10.1021/jacs.4c04068. Epub 2024 Jun 12. J Am Chem Soc. 2024. PMID: 38865257 Free PMC article.
-
Historical and Recent Developments in the Chemistry of Cyanate Congeners.Inorg Chem. 2025 Jul 7;64(26):12900-12917. doi: 10.1021/acs.inorgchem.5c01041. Epub 2025 Jun 11. Inorg Chem. 2025. PMID: 40498324 Free PMC article. Review.
-
Catalytic Amino Group Transfer Reactions Mediated by Photoinduced Nitrene Formation from Rhodium-Hydroxamates.Angew Chem Int Ed Engl. 2025 Apr 7;64(15):e202422461. doi: 10.1002/anie.202422461. Epub 2025 Feb 25. Angew Chem Int Ed Engl. 2025. PMID: 39961777 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

