Tumour necrosis factor inhibitors in Crohn's disease and the effect on surgery rates
- PMID: 34905282
- PMCID: PMC9306633
- DOI: 10.1111/codi.16021
Tumour necrosis factor inhibitors in Crohn's disease and the effect on surgery rates
Abstract
Aim: Surgery is an important therapeutic option for Crohn's disease. The need for first bowel surgery seems to have decreased with the introduction of tumour necrosis factor inhibitors (TNFi; adalimumab or infliximab). However, the impact of TNFi on the need for intestinal surgery in Crohn's disease patients irrespective of prior bowel resection is not known. The aim of this work is to compare the incidence of bowel surgery in Crohn's disease patients who remain on TNFi treatment versus those who discontinue it.
Method: We performed a nationwide register-based observational cohort study in Sweden of all incident and prevalent cases of Crohn's disease who started first-line TNFi treatment between 2006 and 2017. Patients were categorized according to TNFi treatment retention less than or beyond 1 year. The study cohort was evaluated with regard to incidence of bowel surgery from 12 months after the first ever TNFi dispensation.
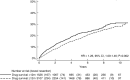
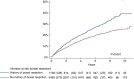
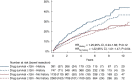
Results: We identified 5003 Crohn's disease patients with TNFi exposure: 3748 surgery naïve and 1255 with bowel surgery prior to TNFi initiation. Of these patients, 7% (n = 353) were subjected to abdominal surgery during the first 12 months after the start of TNFi and were subsequently excluded from the main analysis. A majority (62%) continued TNFi for 12 months or more. Treatment with TNFi for less than 12 months was associated with a significantly higher surgery rate compared with patients who continued on TNFi for 12 months or more (hazard ratio 1.26, 95% CI 1.09-1.46; p = 0.002).
Conclusion: Treatment with TNFi for less than 12 months was associated with a higher risk of bowel surgery in Crohn's disease patients compared with those who continued TNFi for 12 months or more.
Keywords: Crohn's disease; biologics; bowel surgery.
© 2021 The Authors. Colorectal Disease published by John Wiley & Sons Ltd on behalf of Association of Coloproctology of Great Britain and Ireland.
Conflict of interest statement
ME has received honoraria for lectures and consultancy from AbbVie, Merck (MSD), Takeda, Ferring, Orion Pharma, Otsuka, Tillotts, ITH, Novartis, Pfizer and Janssen, and received research funding from AbbVie and MSD. JKS has served as an external consultant to Parexel and Janssen. OO has been Principal Investigator on projects at Karolinska Institutet partly financed by investigator‐initiated grants from Janssen and Ferring, and also reports a grant from Pfizer in the context of a national safety monitoring program. None of those studies have any relation to the present study. Karolinska Institutet also has received fees for OO's lectures and participation on advisory boards from Janssen, Ferring, Takeda and Pfizer regarding topics not related to the present study. PM has received honoraria for lectures from Ferring, AbbVie and Takeda and has served as an external consultant to Janssen and AbbVie and has been Principal Investigator for a research project partly funded by Takeda. CH has received honoraria for lectures from Ferring, AbbVie, Janssen and Takeda and has served as an external consultant to Pfizer. ÅHE has worked on projects at Karolinska Institutet and SWIBREG partly financed by grants from Ferring and Jansen.
Figures

References
-
- Colombel JF, Rutgeerts PJ, Sandborn WJ, Yang M, Camez A, Pollack PF, et al. Adalimumab induces deep remission in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2014;12(3):414–422.e5. - PubMed
-
- Panaccione R, Colombel JF, Sandborn WJ, Rutgeerts P, D'Haens GR, Robinson AM, et al. Adalimumab sustains clinical remission and overall clinical benefit after 2 years of therapy for Crohn's disease. Aliment Pharmacol Ther. 2010;31(12):1296–309. - PubMed
-
- Lichtenstein GR, Yan S, Bala M, Blank M, Sands BE. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn's disease. Gastroenterology. 2005;128(4):862–9. - PubMed
-
- Kalman TD, Everhov ÅH, Nordenvall C, Sachs MC, Halfvarson J, Ekbom A, et al. Decrease in primary but not in secondary abdominal surgery for Crohn's disease: nationwide cohort study, 1990–2014. Br J Surg. 2020;107(11):1529–38. - PubMed

