Controlling Chemoselectivity of Catalytic Hydroboration with Light
- PMID: 34905284
- PMCID: PMC9305532
- DOI: 10.1002/anie.202114482
Controlling Chemoselectivity of Catalytic Hydroboration with Light
Abstract
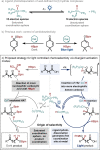
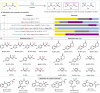
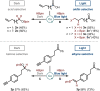
The ability to selectively react one functional group in the presence of another underpins efficient reaction sequences. Despite many designer catalytic systems being reported for hydroboration reactions, which allow introduction of a functional handle for cross-coupling or act as mild method for reducing polar functionality, these platforms rarely deal with more complex systems where multiple potentially reactive sites exist. Here we demonstrate, for the first time, the ability to use light to distinguish between ketones and carboxylic acids in more complex molecules. By taking advantage of different activation modes, a single catalytic system can be used for hydroboration, with the chemoselectivity dictated only by the presence or absence of visible light.
Keywords: Chemoselectivity; Cobalt; Hydroboration; Photochemistry; Reduction.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
References
-
- Mahatthananchai J., Dumas A. M., Bode J. W., Angew. Chem. Int. Ed. 2012, 51, 10954–10990; - PubMed
- Angew. Chem. 2012, 124, 11114–11152.
-
- Hui C., Chen F., Pu F., Xu J., Nat. Rev. Chem. 2019, 3, 85–107.
-
- Beletskaya I. P., Nájera C., Yus M., Chem. Soc. Rev. 2020, 49, 7101–7166. - PubMed
-
- Chatterjee B., Jena S., Chugh V., Weyhermüller T., Werlé C., ACS Catal. 2021, 11, 7176–7185.
Grants and funding
LinkOut - more resources
Full Text Sources

