Regio- and Enantioselective Iridium-Catalyzed Amination of Alkyl-Substituted Allylic Acetates with Secondary Amines
- PMID: 34905364
- PMCID: PMC8764998
- DOI: 10.1021/acs.orglett.1c04135
Regio- and Enantioselective Iridium-Catalyzed Amination of Alkyl-Substituted Allylic Acetates with Secondary Amines
Abstract
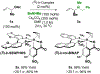
Robust air-stable cyclometalated π-allyliridium C,O-benzoates modified by (S)-tol-BINAP catalyze the reaction of secondary aliphatic amines with racemic alkyl-substituted allylic acetates to furnish products of allylic amination with high levels of enantioselectivity. Complete branched regioselectivities were observed despite the formation of more highly substituted C-N bonds.
Figures


Similar articles
-
Regio- and Enantioselective Iridium-Catalyzed N-Allylation of Indoles and Related Azoles with Racemic Branched Alkyl-Substituted Allylic Acetates.Angew Chem Int Ed Engl. 2019 Jun 3;58(23):7762-7766. doi: 10.1002/anie.201902799. Epub 2019 May 6. Angew Chem Int Ed Engl. 2019. PMID: 30964961 Free PMC article.
-
Amphiphilic π-Allyliridium C,O-Benzoates Enable Regio- and Enantioselective Amination of Branched Allylic Acetates Bearing Linear Alkyl Groups.J Am Chem Soc. 2018 Jan 31;140(4):1275-1279. doi: 10.1021/jacs.7b13482. Epub 2018 Jan 19. J Am Chem Soc. 2018. PMID: 29350523 Free PMC article.
-
Regio- and Enantioselective Iridium-Catalyzed Amination of Racemic Branched Alkyl-Substituted Allylic Acetates with Primary and Secondary Aromatic and Heteroaromatic Amines.J Am Chem Soc. 2019 Jan 9;141(1):671-676. doi: 10.1021/jacs.8b12152. Epub 2018 Dec 20. J Am Chem Soc. 2019. PMID: 30571092 Free PMC article.
-
Recent advances and applications of iridium-catalysed asymmetric allylic substitution.Org Biomol Chem. 2012 Apr 28;10(16):3147-63. doi: 10.1039/c2ob07086c. Epub 2012 Mar 12. Org Biomol Chem. 2012. PMID: 22407450 Review.
-
A Regio- and Stereodivergent Synthesis of Homoallylic Amines by a One-Pot Cooperative-Catalysis-Based Allylic Alkylation/Hofmann Rearrangement Strategy.Angew Chem Int Ed Engl. 2019 Jul 29;58(31):10521-10527. doi: 10.1002/anie.201905426. Epub 2019 Jun 28. Angew Chem Int Ed Engl. 2019. PMID: 31132203 Free PMC article. Review.
Cited by
-
Chiral Amines via Enantioselective π-Allyliridium-C,O-Benzoate-Catalyzed Allylic Alkylation: Student Training via Industrial-Academic Collaboration.Acc Chem Res. 2022 Aug 2;55(15):2138-2147. doi: 10.1021/acs.accounts.2c00302. Epub 2022 Jul 13. Acc Chem Res. 2022. PMID: 35830564 Free PMC article.
-
Scope and Mechanistic Probe into Asymmetric Synthesis of α-Trisubstituted-α-Tertiary Amines by Rhodium Catalysis.J Am Chem Soc. 2023 Sep 13;145(36):19642-19654. doi: 10.1021/jacs.3c04211. Epub 2023 Aug 31. J Am Chem Soc. 2023. PMID: 37651695 Free PMC article.
-
Kinetic, ESI-CID-MS and Computational Studies of π-Allyliridium C,O-Benzoate-Catalyzed Allylic Amination: Understanding the Effect of Cesium Ion.ACS Catal. 2022 Mar 18;12(6):3660-3668. doi: 10.1021/acscatal.2c00470. Epub 2022 Mar 8. ACS Catal. 2022. PMID: 36092640 Free PMC article.
References
-
- The initial reports by Tsuji and Trost involved the addition of nucleophiles to stoichiometric quantities of preformed π-allylpalladium chloride dimers:Tsuji J; Takahashi H; Morikawa M Organic Synthesis by Noble Metal Compounds. XVII. Reaction of π-Allylpalladium Chloride with Nucleophiles. Tetrahedron Lett. 1965, 6, 4387–4388.
- Trost BM; Fullerton TJ New Synthetic Reactions. Allylic Alkylation. J. Am. Chem. Soc 1973, 95, 292–294.
-
- For selected reviews on palladium-catalyzed allylic alkylation, see:Trost BM; Van Vranken DL Asymmetric Transition Metal-Catalyzed Allylic Alkylations. Chem. Rev 1996, 96, 395–422. - PubMed
- Trost BM; Crawley ML Asymmetric Transition-Metal-Catalyzed Allylic Alkylations: Applications in Total Synthesis. Chem. Rev 2003, 103, 2921–2944. - PubMed
- Pritchett BP; Stoltz BM Enantioselective Palladium-Catalyzed Allylic Alkylation Reactions in The Synthesis of Aspidosperma and Structurally Related Monoterpene Indole Alkaloids. Nat. Prod. Rep 2018, 35, 559–574. - PubMed
- Trost BM; Schultz JE Palladium-Catalyzed Asymmetric Allylic Alkylation Strategies for the Synthesis of Acyclic Tetrasubstituted Stereocenters. Synthesis 2019, 51, 1–30. - PubMed
- James Jinju; Jackson Mark; Guiry Patrick J. Palladium-Catalyzed Decarboxylative Asymmetric Allylic Alkylation: Development, Mechanistic Understanding and Recent Advances. Adv. Synth. Catal 2019, 361, 3016–3049. - PubMed
-
- For a recent review, see: Cheng Q; Tu H-F; Zheng C; Qu J-P; Helmchen G; You S-L Iridium-Catalyzed Asymmetric Allylic Substitution Reactions. Chem. Rev 2019, 119, 1855–1969. - PubMed
-
- Takeuchi R; Ue N; Tanabe K; Yamashita K; Shiga N Iridium Complex-Catalyzed Allylic Amination of Allylic Esters. J. Am. Chem. Soc 2001, 123, 9525–9534. - PubMed
- Onodera G; Watabe K; Matsubara M; Oda K; Kezuka S; Takeuchi R Iridium-Catalyzed Enantioselective Allylic Alkylation using Chiral Phosphoramidite Ligand Bearing an Amide Moiety. Adv. Synth. Catal 2008, 2725–2732. - PubMed
-
- Review: Takeuchi R; Kezuka S Iridium-Catalyzed Formation of Carbon-Carbon and Carbon-Heteroatom Bonds. Synthesis 2006, 3349–3366.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

