Side Chain Conformation and Its Influence on Glycosylation Selectivity in Hexo- and Higher Carbon Furanosides
- PMID: 34905382
- PMCID: PMC8741747
- DOI: 10.1021/acs.joc.1c02374
Side Chain Conformation and Its Influence on Glycosylation Selectivity in Hexo- and Higher Carbon Furanosides
Abstract
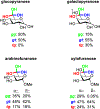
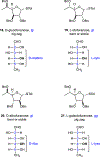
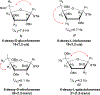
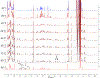
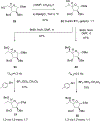
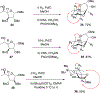
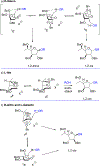
We describe the synthesis and side chain conformational analysis of a series of four 6-deoxy-2,3,5-tri-O-benzyl hexofuranosyl donors with the d-gluco, l-ido, d-altro, and l-galacto configurations. The conformation of the exocyclic bond of these compounds depends on the relative configuration of the point of attachment of the side chain to the ring and of the two flanking centers and can be predicted on that basis analogously to the heptopyranose analogs. Variable-temperature nuclear magnetic resonance (VT NMR) spectroscopy of the activated donors reveals complex, configuration-dependent mixtures of intermediates that we interpret in terms of fused and bridged oxonium ions arising from participation by the various benzyl ethers. The increased importance of ether participation in the furanoside series compared to the pyranosides is discussed in terms of the reduced stabilization afforded to furanosyl oxocarbenium ions by covalent triflate formation. The stereoselectivities of the four donors are discussed on the basis of the benzyl ether participation model.
Figures



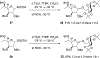
Similar articles
-
Influence of Configuration at the 4- and 6-Positions on the Conformation and Anomeric Reactivity and Selectivity of 7-Deoxyheptopyranosyl Donors: Discovery of a Highly Equatorially Selective l-glycero-d-gluco-Heptopyranosyl Donor.J Org Chem. 2021 Sep 3;86(17):12199-12225. doi: 10.1021/acs.joc.1c01535. Epub 2021 Aug 3. J Org Chem. 2021. PMID: 34343001 Free PMC article.
-
(13)C-NMR glycosylation effects in (1→3)-linked furanosyl-pyranosides.Carbohydr Res. 2015 Nov 19;417:1-10. doi: 10.1016/j.carres.2015.08.014. Epub 2015 Aug 28. Carbohydr Res. 2015. PMID: 26382080
-
Stereoselective C-glycoside formation with 2-O-benzyl-4,6-O-benzylidene protected 3-deoxy gluco- and mannopyranoside donors: comparison with O-glycoside formation.J Org Chem. 2012 Oct 19;77(20):8905-12. doi: 10.1021/jo3011655. Epub 2012 Oct 11. J Org Chem. 2012. PMID: 23009024 Free PMC article.
-
Design of chemical glycosyl donors: does changing ring conformation influence selectivity/reactivity?Chem Soc Rev. 2013 May 21;42(10):4297-309. doi: 10.1039/c3cs35457a. Epub 2013 Jan 30. Chem Soc Rev. 2013. PMID: 23364773 Review.
-
Glycosyl Oxocarbenium Ions: Structure, Conformation, Reactivity, and Interactions.Acc Chem Res. 2021 Jun 1;54(11):2552-2564. doi: 10.1021/acs.accounts.1c00021. Epub 2021 Apr 30. Acc Chem Res. 2021. PMID: 33930267 Free PMC article. Review.
Cited by
-
Can Side-Chain Conformation and Glycosylation Selectivity of Hexopyranosyl Donors Be Controlled with a Dummy Ligand?J Org Chem. 2023 Mar 17;88(6):3678-3696. doi: 10.1021/acs.joc.2c02889. Epub 2023 Mar 6. J Org Chem. 2023. PMID: 36877600 Free PMC article.
-
Comparison of glycosyl donors: a supramer approach.Beilstein J Org Chem. 2024 Jan 31;20:181-192. doi: 10.3762/bjoc.20.18. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38318458 Free PMC article.
-
Involvement of an Oxonium Ion Intermediate in Controlling the Diastereoselectivity of Nucleophilic Substitution Reactions of Septanoses.Org Lett. 2023 Jan 13;25(1):152-157. doi: 10.1021/acs.orglett.2c03963. Epub 2023 Jan 4. Org Lett. 2023. PMID: 36599094 Free PMC article.
-
Syntheses of Legionaminic Acid, Pseudaminic Acid, Acetaminic Acid, 8-epi-Acetaminic Acid, and 8-epi-Legionaminic Acid Glycosyl Donors from N-Acetylneuraminic Acid by Side Chain Exchange.Org Lett. 2022 Apr 29;24(16):2998-3002. doi: 10.1021/acs.orglett.2c00894. Epub 2022 Apr 14. Org Lett. 2022. PMID: 35420827 Free PMC article.
-
Unraveling the promoter effect and the roles of counterion exchange in glycosylation reaction.Sci Adv. 2023 Oct 20;9(42):eadk0531. doi: 10.1126/sciadv.adk0531. Epub 2023 Oct 18. Sci Adv. 2023. PMID: 37851803 Free PMC article.
References
-
- Jensen HH; Nordstrøm LU; Bols M, The Disarming Effect of the 4,6-Acetal Group on Glycoside Reactivity: Torsional or Electronic. J. Am. Chem. Soc 2004, 126, 9205–92132. - PubMed
-
- Rao VSR; Qasba PK; Balaji PV; Chandrasekaran R, Conformation of Carbohydrates. Harwood Academic Publishers: Amsterdam, 1998; p 359.
-
- Bock K; Duus JO, A Conformational Study of Hydroxymethyl Groups in Carbohydrates Investigated by 1H NMR Spectroscopy. J. Carbohydr. Chem 1994, 13, 513–543.
-
- Grindley TB, Structure and Conformation of Carbohydrates. In Glycoscience: Chemistry and Chemical Biology, Fraser-Reid B; Tatsuta K; Thiem J, Eds. Springer: Berlin, 2001; Vol. 1, pp 3–51.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

