Use of Communication Technology to Improve Clinical Trial Participation in Adolescents and Young Adults With Cancer: Consensus Statement From the Children's Oncology Group Adolescent and Young Adult Responsible Investigator Network
- PMID: 34905405
- PMCID: PMC8932547
- DOI: 10.1200/OP.21.00554
Use of Communication Technology to Improve Clinical Trial Participation in Adolescents and Young Adults With Cancer: Consensus Statement From the Children's Oncology Group Adolescent and Young Adult Responsible Investigator Network
Abstract
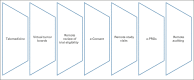
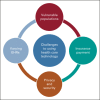
Adolescents and young adults (AYAs; age 15-39 years) with cancer are under-represented in cancer clinical trials because of patient, provider, and institutional barriers. Health care technology is increasingly available to and highly used among AYAs and has the potential to improve cancer care delivery. The COVID-19 pandemic forced institutions to rapidly adopt novel approaches for enrollment and monitoring of patients on cancer clinical trials, many of which have the potential for improving AYA trial participation overall. This consensus statement from the Children's Oncology Group AYA Oncology Discipline Committee reviews opportunities to use technology to optimize AYA trial enrollment and study conduct, as well as considerations for widespread implementation of these practices. The use of remote patient eligibility screening, electronic informed consent, virtual tumor boards, remote study visits, and remote patient monitoring are recommended to increase AYA access to trials and decrease the burden of participation. Widespread adoption of these strategies will require new policies focusing on reimbursement for telehealth, license portability, facile communication between electronic health record systems and advanced safeguards to maintain patient privacy and security. Studies are needed to determine optimal approaches to further incorporate technology at every stage of the clinical trial process, from enrollment through study completion.
Conflict of interest statement
Figures
Similar articles
-
Utilization of Teleconsent for Adolescent and Young Adult Cancer Clinical Trials, a Report from the Children's Oncology Group.J Adolesc Young Adult Oncol. 2024 Feb;13(1):132-137. doi: 10.1089/jayao.2023.0085. Epub 2023 Aug 4. J Adolesc Young Adult Oncol. 2024. PMID: 37540127 Free PMC article.
-
Shared barriers and facilitators to enrollment of adolescents and young adults on cancer clinical trials.Sci Rep. 2022 Mar 9;12(1):3875. doi: 10.1038/s41598-022-07703-5. Sci Rep. 2022. PMID: 35264642 Free PMC article.
-
The Children's Oncology Group Adolescent and Young Adult Responsible Investigator Network: A New Model for Addressing Site-Level Factors Impacting Clinical Trial Enrollment.J Adolesc Young Adult Oncol. 2020 Aug;9(4):522-527. doi: 10.1089/jayao.2019.0139. Epub 2020 Feb 20. J Adolesc Young Adult Oncol. 2020. PMID: 32077782 Free PMC article.
-
Systematic review of barriers and facilitators to clinical trial enrollment among adolescents and young adults with cancer: Identifying opportunities for intervention.Cancer. 2020 Mar 1;126(5):949-957. doi: 10.1002/cncr.32675. Epub 2019 Dec 23. Cancer. 2020. PMID: 31869454 Free PMC article.
-
Adolescent angst: enrollment on clinical trials.Hematology Am Soc Hematol Educ Program. 2018 Nov 30;2018(1):154-160. doi: 10.1182/asheducation-2018.1.154. Hematology Am Soc Hematol Educ Program. 2018. PMID: 30504304 Free PMC article. Review.
Cited by
-
Insights From Diverse Perspectives on Social Media Messages to Inform Young Adults With Cancer About Clinical Trials: Focus Group Study.JMIR Form Res. 2025 Jan 20;9:e64265. doi: 10.2196/64265. JMIR Form Res. 2025. PMID: 39864817 Free PMC article.
-
Children's Oncology Group 2023 blueprint for research: Adolescent and young adult oncology.Pediatr Blood Cancer. 2023 Sep;70 Suppl 6(Suppl 6):e30564. doi: 10.1002/pbc.30564. Epub 2023 Jul 13. Pediatr Blood Cancer. 2023. PMID: 37439574 Free PMC article.
-
Sharing is caring: a network collaborative approach to identify and address barriers in accessing clinical trials in adolescents and young adults with leukemia and lymphoma.Hematology Am Soc Hematol Educ Program. 2024 Dec 6;2024(1):27-33. doi: 10.1182/hematology.2024000526. Hematology Am Soc Hematol Educ Program. 2024. PMID: 39643982 Free PMC article. Review.
-
Challenges to successful outcomes in AYAs with ALL and potential solutions.Hematology Am Soc Hematol Educ Program. 2023 Dec 8;2023(1):587-592. doi: 10.1182/hematology.2023000512. Hematology Am Soc Hematol Educ Program. 2023. PMID: 38066918 Free PMC article.
-
Perspectives on Conducting Behavioral Intervention With Adolescents Using a Virtual Platform-A Thematic Analysis From the Viewpoint of Study Coordinators.Pediatr Transplant. 2025 Feb;29(1):e70006. doi: 10.1111/petr.70006. Pediatr Transplant. 2025. PMID: 39776082 Free PMC article.
References
-
- Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer. Report of the Adolescent and Young Adult Oncology Progress Review Group. https://www.livestrong.org/sites/default/files/what-we-do/reports/ayao_p...
-
- Liu L, Krailo M, Reaman GH, et al. : Childhood cancer patients' access to cooperative group cancer programs: A population-based study. Cancer 97:1339-1345, 2003 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

