Nuclear receptor binding SET domain protein 1 promotes epithelial-mesenchymal transition in paclitaxel-resistant breast cancer cells via regulating nuclear factor kappa B and F-box and leucine-rich repeat protein 11
- PMID: 34905470
- PMCID: PMC8810193
- DOI: 10.1080/21655979.2021.2009963
Nuclear receptor binding SET domain protein 1 promotes epithelial-mesenchymal transition in paclitaxel-resistant breast cancer cells via regulating nuclear factor kappa B and F-box and leucine-rich repeat protein 11
Abstract
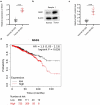
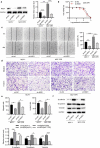
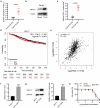
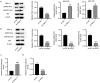
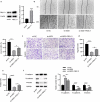
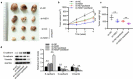
Breast cancer (BC) is regarded as the major cause of cancer-associated deaths in women. Paclitaxel exerts a critical impact on the chemotherapy of BC, but the resistance to paclitaxel becomes a great obstacle in treating the disease. It is reported that noncoding RNA nuclear receptor binding SET domain protein 1 (NSD1) plays a significant role in drug resistance; however, the special role of NSD1 in paclitaxel-resistant BC is unclear. Human BC cell line MCF-7 was used to establish paclitaxel-resistant BC cells (MCF-7/PR). Reverse transcription quantitative polymerase chain reaction (RT-qPCR) displayed that NSD1 and F-box and leucine-rich repeat protein 11 (FBXL11) were highly expressed in BC tissues. Western blotting was utilized for protein level assessment. Cell counting kit-8 (CCK-8), Transwell, wound healing assays, and animal experiments were conducted to examine the influence of NSD1 or FBXL11 on the malignant behaviors of BC in vitro and in vivo, respectively. Transfected MCF-7/PR cells were injected subcutaneously into BALB/c nude mice with or without treatment of paclitaxel. The nuclear factor kappa B (NF-kB) activity was evaluated by the luciferase reporter assay. Results showed that NSD1 knockdown inhibited the epithelial-mesenchymal transition (EMT), migration and invasiveness of BC in vitro, which was rescued by FBXL11 overexpression. Furthermore, NSD1 silencing promoted paclitaxel sensitivity of paclitaxel-resistant BC cells and suppressed tumor growth and paclitaxel resistance in vivo. NSD1 knockdown reduced NF-kB activity, while FBXL11 inhibition markedly increased NF-kB activity. Collectively, NSD1 facilitates the EMT, migration and invasion in paclitaxel-resistant BC cells via regulating NF-kB and FBXL11.
Keywords: Breast cancer; FBXL11; NF-KB; NSD1; paclitaxel.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
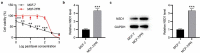
Similar articles
-
Knockdown of nuclear receptor binding SET domain-containing protein 1 (NSD1) inhibits proliferation and facilitates apoptosis in paclitaxel-resistant breast cancer cells via inactivating the Wnt/β-catenin signaling pathway.Bioengineered. 2022 Feb;13(2):3526-3536. doi: 10.1080/21655979.2021.2018973. Bioengineered. 2022. PMID: 35200072 Free PMC article.
-
Regulation of NF-kappaB by NSD1/FBXL11-dependent reversible lysine methylation of p65.Proc Natl Acad Sci U S A. 2010 Jan 5;107(1):46-51. doi: 10.1073/pnas.0912493107. Epub 2009 Dec 22. Proc Natl Acad Sci U S A. 2010. PMID: 20080798 Free PMC article.
-
All-trans retinoic acid reverses epithelial-mesenchymal transition in paclitaxel-resistant cells by inhibiting nuclear factor kappa B and upregulating gap junctions.Cancer Sci. 2019 Jan;110(1):379-388. doi: 10.1111/cas.13855. Epub 2018 Nov 27. Cancer Sci. 2019. PMID: 30375704 Free PMC article.
-
Circ-RNF111 contributes to paclitaxel resistance in breast cancer by elevating E2F3 expression via miR-140-5p.Thorac Cancer. 2020 Jul;11(7):1891-1903. doi: 10.1111/1759-7714.13475. Epub 2020 May 23. Thorac Cancer. 2020. PMID: 32445273 Free PMC article.
-
KDM5 family as therapeutic targets in breast cancer: Pathogenesis and therapeutic opportunities and challenges.Mol Cancer. 2024 May 20;23(1):109. doi: 10.1186/s12943-024-02011-0. Mol Cancer. 2024. PMID: 38769556 Free PMC article. Review.
Cited by
-
Ultrasound and magnetic resonance imaging of cyclic arginine glycine aspartic acid-gadopentetic acid-polylactic acid in human breast cancer by targeting αvβ3 in xenograft-bearing nude mice.Bioengineered. 2022 Mar;13(3):7105-7117. doi: 10.1080/21655979.2022.2045832. Bioengineered. 2022. PMID: 35259049 Free PMC article.
-
Curcumol enhances cisplatin sensitivity of gastric cancer: involvement of microRNA-7 and the nuclear factor-kappa B/snail family transcriptional repressor 1 axis.Bioengineered. 2022 May;13(5):11668-11683. doi: 10.1080/21655979.2022.2070975. Bioengineered. 2022. PMID: 35510522 Free PMC article.
-
Comprehensive Analysis of the Prognostic Value and Molecular Function of CRNDE in Glioma at Bulk and Single-Cell Levels.Cells. 2022 Nov 18;11(22):3669. doi: 10.3390/cells11223669. Cells. 2022. PMID: 36429098 Free PMC article.
-
Structural and functional specificity of H3K36 methylation.Epigenetics Chromatin. 2022 May 18;15(1):17. doi: 10.1186/s13072-022-00446-7. Epigenetics Chromatin. 2022. PMID: 35581654 Free PMC article. Review.
-
NSD Overexpression in the Fat Body Increases Antimicrobial Peptide Production by the Immune Deficiency Pathway in Drosophila.Int J Mol Sci. 2023 May 8;24(9):8443. doi: 10.3390/ijms24098443. Int J Mol Sci. 2023. PMID: 37176149 Free PMC article.
References
-
- Kolak A, Kamińska M, Sygit K, et al. Primary and secondary prevention of breast cancer. Ann Agric Environ Med. 2017. Dec 23;24(4):549–553. - PubMed
-
- Fahad Ullah M. Breast Cancer: current Perspectives on the Disease Status. Adv Exp Med Biol. 2019;1152:51–64. - PubMed
-
- Marupudi NI, Han JE, Li KW, et al. Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin Drug Saf. 2007. Sep;6(5):609–621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
