IGFBP-6/sonic hedgehog/TLR4 signalling axis drives bone marrow fibrotic transformation in primary myelofibrosis
- PMID: 34905501
- PMCID: PMC8714138
- DOI: 10.18632/aging.203779
IGFBP-6/sonic hedgehog/TLR4 signalling axis drives bone marrow fibrotic transformation in primary myelofibrosis
Abstract
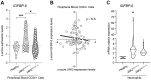
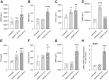
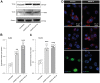
Primary myelofibrosis is a Ph-negative chronic myeloproliferative neoplasm characterized by bone marrow fibrosis and associated with the involvement of several pathways, in addition to bone marrow microenvironment alterations, mostly driven by the activation of the cytokine receptor/JAK2 pathway. Identification of driver mutations has led to the development of targeted therapy for myelofibrosis, contributing to reducing inflammation, although this currently does not translate into bone marrow fibrosis remission. Therefore, understanding the clear molecular cut underlying this pathology is now necessary to improve the clinical outcome of patients. The present study aims to investigate the involvement of IGFBP-6/sonic hedgehog /Toll-like receptor 4 axis in the microenvironment alterations of primary myelofibrosis. We observed a significant increase in IGFBP-6 expression levels in primary myelofibrosis patients, coupled with a reduction to near-normal levels in primary myelofibrosis patients with JAK2V617F mutation. We also found that both IGFBP-6 and purmorphamine, a SHH activator, were able to induce mesenchymal stromal cells differentiation with an up-regulation of cancer-associated fibroblasts markers. Furthermore, TLR4 signaling was also activated after IGFBP-6 and purmorphamine exposure and reverted by cyclopamine exposure, an inhibitor of the SHH pathway, confirming that SHH is involved in TLR4 activation and microenvironment alterations. In conclusion, our results suggest that the IGFBP-6/SHH/TLR4 axis is implicated in alterations of the primary myelofibrosis microenvironment and that IGFBP-6 may play a central role in activating SHH pathway during the fibrotic process.
Keywords: IGFBP-6; MPNs; TLR4; mesenchymal stem cells; myelofibrosis.
Conflict of interest statement
Figures



Similar articles
-
IGFBP-6 Alters Mesenchymal Stromal Cell Phenotype Driving Dasatinib Resistance in Chronic Myeloid Leukemia.Life (Basel). 2023 Jan 17;13(2):259. doi: 10.3390/life13020259. Life (Basel). 2023. PMID: 36836615 Free PMC article.
-
Characterization of the TGF-β1 signaling abnormalities in the Gata1low mouse model of myelofibrosis.Blood. 2013 Apr 25;121(17):3345-63. doi: 10.1182/blood-2012-06-439661. Epub 2013 Mar 5. Blood. 2013. PMID: 23462118 Free PMC article.
-
Sonic hedgehog signaling regulates the expression of insulin-like growth factor binding protein-6 during fetal prostate development.Dev Dyn. 2005 Jul;233(3):829-36. doi: 10.1002/dvdy.20414. Dev Dyn. 2005. PMID: 15906375
-
Bone marrow fibrosis in myelofibrosis: pathogenesis, prognosis and targeted strategies.Haematologica. 2016 Jun;101(6):660-71. doi: 10.3324/haematol.2015.141283. Haematologica. 2016. PMID: 27252511 Free PMC article. Review.
-
IGFBP-6: At the Crossroads of Immunity, Tissue Repair and Fibrosis.Int J Mol Sci. 2022 Apr 14;23(8):4358. doi: 10.3390/ijms23084358. Int J Mol Sci. 2022. PMID: 35457175 Free PMC article. Review.
Cited by
-
ACVR1: A Novel Therapeutic Target to Treat Anemia in Myelofibrosis.Cancers (Basel). 2023 Dec 28;16(1):154. doi: 10.3390/cancers16010154. Cancers (Basel). 2023. PMID: 38201581 Free PMC article. Review.
-
Fibrosis and bone marrow: understanding causation and pathobiology.J Transl Med. 2023 Oct 9;21(1):703. doi: 10.1186/s12967-023-04393-z. J Transl Med. 2023. PMID: 37814319 Free PMC article. Review.
-
Cancer-associated fibroblasts in hematologic malignancies: elucidating roles and spotlighting therapeutic targets.Front Oncol. 2023 Sep 6;13:1193978. doi: 10.3389/fonc.2023.1193978. eCollection 2023. Front Oncol. 2023. PMID: 37746306 Free PMC article. Review.
-
Mesenchymal stromal cells in tumor microenvironment remodeling of BCR-ABL negative myeloproliferative diseases.Front Oncol. 2023 Feb 23;13:1141610. doi: 10.3389/fonc.2023.1141610. eCollection 2023. Front Oncol. 2023. PMID: 36910610 Free PMC article. Review.
-
Cancer associated fibroblasts in tumors: focusing on solid tumors and hematological malignancies.Front Oncol. 2025 Jun 23;15:1596947. doi: 10.3389/fonc.2025.1596947. eCollection 2025. Front Oncol. 2025. PMID: 40626005 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

