Embryonic osteocalcin signaling determines lifelong adrenal steroidogenesis and homeostasis in the mouse
- PMID: 34905510
- PMCID: PMC8843753
- DOI: 10.1172/JCI153752
Embryonic osteocalcin signaling determines lifelong adrenal steroidogenesis and homeostasis in the mouse
Abstract
Through their ability to regulate gene expression in most organs, glucocorticoid (GC) hormones influence numerous physiological processes and are therefore key regulators of organismal homeostasis. In bone, GC hormones inhibit expression of the hormone Osteocalcin for poorly understood reasons. Here, we show that in a classical endocrine feedback loop, osteocalcin in return enhanced the biosynthesis of GC as well as mineralocorticoid hormones (adrenal steroidogenesis) in rodents and primates. Conversely, inactivation of osteocalcin signaling in adrenal glands significantly impaired adrenal growth and steroidogenesis in mice. Embryo-made osteocalcin was necessary for normal Sf1 expression in fetal adrenal cells and adrenal cell steroidogenic differentiation and therefore determined the number of steroidogenic cells present in the adrenal glands of adult animals. Embryonic, not postnatal, osteocalcin also governed adrenal growth, adrenal steroidogenesis, blood pressure, electrolyte equilibrium, and the rise in circulating corticosterone levels during the acute stress response in adult offspring. This osteocalcin-dependent regulation of adrenal development and steroidogenesis occurred even in the absence of a functional hypothalamus/pituitary/adrenal axis and explains why osteocalcin administration during pregnancy promoted adrenal growth and steroidogenesis and improved the survival of adrenocorticotropic hormone signaling-deficient animals. This study reveals that a bone-derived embryonic hormone influences lifelong adrenal functions and organismal homeostasis in the mouse.
Keywords: Bone Biology; Metabolism; Mouse models.
Conflict of interest statement
Figures
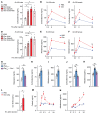
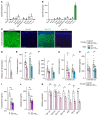
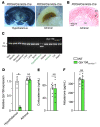





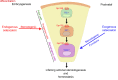
Comment in
-
Osteocalcin influences lifelong adrenal function.Nat Rev Endocrinol. 2022 Mar;18(3):135. doi: 10.1038/s41574-021-00632-9. Nat Rev Endocrinol. 2022. PMID: 34992234 No abstract available.
-
Bones and adrenal organogenesis: how embryonic osteocalcin influences lifelong adrenal function.J Clin Invest. 2022 Feb 15;132(4):e157200. doi: 10.1172/JCI157200. J Clin Invest. 2022. PMID: 35166237 Free PMC article.
Similar articles
-
Control and ontogeny of hypothalamic-pituitary-adrenal function in the fetal rat.J Dev Physiol. 1989 Jul;12(1):1-9. J Dev Physiol. 1989. PMID: 2559115 Review. No abstract available.
-
Osteocalcin of maternal and embryonic origins synergize to establish homeostasis in offspring.EMBO Rep. 2024 Feb;25(2):593-615. doi: 10.1038/s44319-023-00031-3. Epub 2024 Jan 16. EMBO Rep. 2024. PMID: 38228788 Free PMC article.
-
Periconceptional ethanol exposure alters hypothalamic-pituitary-adrenal axis function, signalling elements and associated behaviours in a rodent model.Psychoneuroendocrinology. 2020 Dec;122:104901. doi: 10.1016/j.psyneuen.2020.104901. Epub 2020 Oct 7. Psychoneuroendocrinology. 2020. PMID: 33070024
-
Periconceptional ethanol exposure alters the stress axis in adult female but not male rat offspring.Stress. 2019 May;22(3):347-357. doi: 10.1080/10253890.2018.1563068. Epub 2019 Feb 11. Stress. 2019. PMID: 30741061
-
[Mechanisms of adrenal embryogenesis].Usp Fiziol Nauk. 2001 Apr-Jun;32(2):99-112. Usp Fiziol Nauk. 2001. PMID: 11548594 Review. Russian.
Cited by
-
The emerging roles of GPR158 in the regulation of the endocrine system.Front Cell Dev Biol. 2022 Nov 18;10:1034348. doi: 10.3389/fcell.2022.1034348. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36467406 Free PMC article. Review.
-
Osteocalcin: A Multifaceted Bone-Derived Hormone.Annu Rev Nutr. 2023 Aug 21;43:55-71. doi: 10.1146/annurev-nutr-061121-091348. Annu Rev Nutr. 2023. PMID: 37603430 Free PMC article. Review.
-
Triple-gene deletion for osteocalcin significantly impairs the alignment of hydroxyapatite crystals and collagen in mice.Front Physiol. 2023 Mar 28;14:1136561. doi: 10.3389/fphys.2023.1136561. eCollection 2023. Front Physiol. 2023. PMID: 37057181 Free PMC article.
-
The role of osteocalcin in regulating the acute stress response.Front Pharmacol. 2025 Jul 14;16:1646558. doi: 10.3389/fphar.2025.1646558. eCollection 2025. Front Pharmacol. 2025. PMID: 40727110 Free PMC article. Review.
-
Osteocalcin and the physiology of danger.FEBS Lett. 2022 Mar;596(5):665-680. doi: 10.1002/1873-3468.14259. Epub 2022 Jan 21. FEBS Lett. 2022. PMID: 34913486 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

