TM2D genes regulate Notch signaling and neuronal function in Drosophila
- PMID: 34905536
- PMCID: PMC8714088
- DOI: 10.1371/journal.pgen.1009962
TM2D genes regulate Notch signaling and neuronal function in Drosophila
Abstract
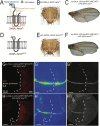
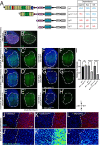
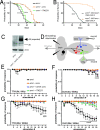
TM2 domain containing (TM2D) proteins are conserved in metazoans and encoded by three separate genes in each model organism species that has been sequenced. Rare variants in TM2D3 are associated with Alzheimer's disease (AD) and its fly ortholog almondex is required for embryonic Notch signaling. However, the functions of this gene family remain elusive. We knocked-out all three TM2D genes (almondex, CG11103/amaretto, CG10795/biscotti) in Drosophila and found that they share the same maternal-effect neurogenic defect. Triple null animals are not phenotypically worse than single nulls, suggesting these genes function together. Overexpression of the most conserved region of the TM2D proteins acts as a potent inhibitor of Notch signaling at the γ-secretase cleavage step. Lastly, Almondex is detected in the brain and its loss causes shortened lifespan accompanied by progressive motor and electrophysiological defects. The functional links between all three TM2D genes are likely to be evolutionarily conserved, suggesting that this entire gene family may be involved in AD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
TM2D3, a mammalian homologue of Drosophila neurogenic gene product Almondex, regulates surface presentation of Notch receptors.Sci Rep. 2023 Nov 27;13(1):20913. doi: 10.1038/s41598-023-46866-7. Sci Rep. 2023. PMID: 38016980 Free PMC article.
-
Maternal almondex, a neurogenic gene, is required for proper subcellular Notch distribution in early Drosophila embryogenesis.Dev Growth Differ. 2020 Jan;62(1):80-93. doi: 10.1111/dgd.12639. Epub 2019 Nov 28. Dev Growth Differ. 2020. PMID: 31782145
-
Implication of the Drosophila beta-amyloid peptide binding-like protein AMX in Notch signaling during early neurogenesis.Brain Res Bull. 2008 Mar 18;75(2-4):305-9. doi: 10.1016/j.brainresbull.2007.10.060. Epub 2007 Dec 3. Brain Res Bull. 2008. PMID: 18331889
-
Comparative aspects of Notch signaling in lower and higher eukaryotes.Perspect Dev Neurobiol. 1997;4(4):325-43. Perspect Dev Neurobiol. 1997. PMID: 9171446 Review.
-
Making sense out of missense mutations: Mechanistic dissection of Notch receptors through structure-function studies in Drosophila.Dev Growth Differ. 2020 Jan;62(1):15-34. doi: 10.1111/dgd.12640. Epub 2020 Jan 13. Dev Growth Differ. 2020. PMID: 31943162 Free PMC article. Review.
Cited by
-
Patterns and drivers of 43,617 mosaic chromosomal alterations in blood.medRxiv [Preprint]. 2025 Jul 30:2025.07.30.25332451. doi: 10.1101/2025.07.30.25332451. medRxiv. 2025. PMID: 40766159 Free PMC article. Preprint.
-
Functional Studies of Genetic Variants Associated with Human Diseases in Notch Signaling-Related Genes Using Drosophila.Methods Mol Biol. 2022;2472:235-276. doi: 10.1007/978-1-0716-2201-8_19. Methods Mol Biol. 2022. PMID: 35674905 Free PMC article.
-
Bi-allelic variants in TM2D3 cause a severe syndromic neurodevelopmental disorder associated with endoplasmic reticulum and mitochondrial abnormalities.Am J Hum Genet. 2025 Jul 3;112(7):1711-1721. doi: 10.1016/j.ajhg.2025.05.004. Epub 2025 May 30. Am J Hum Genet. 2025. PMID: 40449487
-
Notch Signalling Under Maternal-to-Zygotic Transition.Fly (Austin). 2022 Dec;16(1):347-359. doi: 10.1080/19336934.2022.2139981. Fly (Austin). 2022. PMID: 36346359 Free PMC article. Review.
-
Identification of Protein Biomarkers for Differentiating Listeria monocytogenes Genetic Lineage III.Foods. 2024 Apr 24;13(9):1302. doi: 10.3390/foods13091302. Foods. 2024. PMID: 38731673 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

